1. Write the balanced net ionic equation for the reaction between MnO4" ion and Fe2+ ion in acid solution. 2. How many moles of Fe2* ion can be oxidized by 1.2 x 10-2 moles MnO4 ion in the reaction in Question 1?
1. Write the balanced net ionic equation for the reaction between MnO4" ion and Fe2+ ion in acid solution. 2. How many moles of Fe2* ion can be oxidized by 1.2 x 10-2 moles MnO4 ion in the reaction in Question 1?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 147CP: The measurement of pH using a glass electrode obeys the Nernst equation. The typical response of a...
Related questions
Question
How many moles of Fe^2+ ion can be oxidized by 1.2 ×10^-2 moles MnO4 ion in the reaction between MnO4 ion and Fe^2+

Transcribed Image Text:lab, complete items a, b, c, and d (title, purpose, chemicals and
equipment, and summary of procedure) as described on page 10 of Exp. 1 on an
8 1/2 x 11 sheet of paper.
Answer the following questions on 8 1/2 x 11 sheet of paper or in your laboratory
notebook if one is required by your instructor:
1. Write the balanced net ionic equation for the reaction between MnO4 ion and
Fe2+ ion in acid solution.
2. How many moles of Fe2+ ion can be oxidized by 1.2 x 10-2 moles MnO4 ion in the
reaction in Question 1?
3. A solid sample containing some Fe2* ion weighs 1.923 g. It requires 36.44 mL
0.0244 M KMNO4 to titrate the Fe2* in the dissolved sample to a pink end point.
a. How many moles MnO4 ion are required?
b. How many moles Fe2* are there in the sample?
C. How many grams of iron are there in the sample?
d. What is the percentage of Fe in the sample?
Lab Activities:
Go over the sample calculations and prelab questions with your lab instructor
• Complete lab and fill in data sheet for the Standardization of Potassium
Permanganate. The next data sheet, Percent Iron in Unknown Sample will be
completed the following lab period.
1 |P age
Expert Solution
Step 1
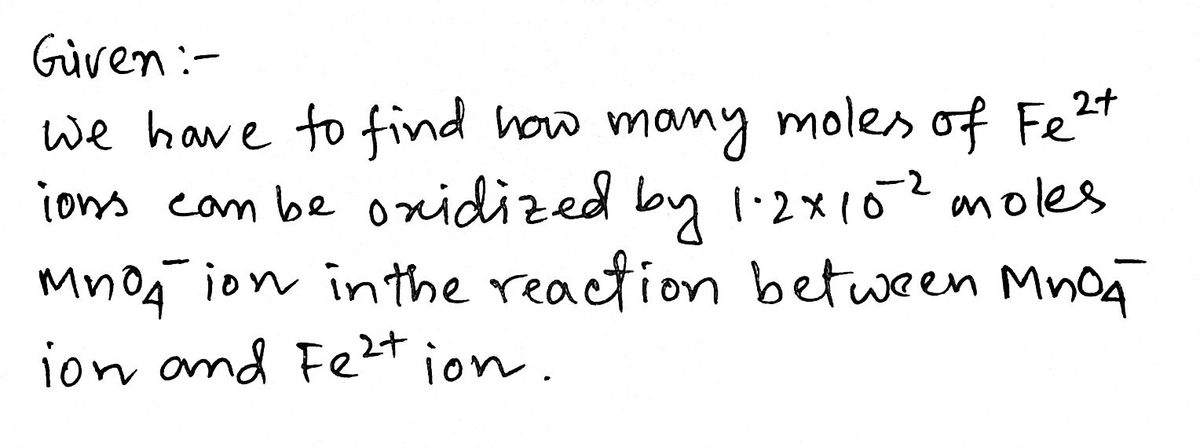
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax