1.00-mol sample of a monatomic ideal gas is taken through e cycle shown in the figure. At point A, the pressure, volume, d temperature are P¡ , Vị , and T; , respectively. In terms of R d T¡ , find the total energy entering the system by heat per cycle, the total energy leaving the system by heat per cycle, the efficiency of an engine operating in this cycle, and the efficiency of an engine operating in a Carnot cycle Iween the same temperature extremes P В ЗР, Qi Q3 2P; P; D Q4 V V; 2V;
1.00-mol sample of a monatomic ideal gas is taken through e cycle shown in the figure. At point A, the pressure, volume, d temperature are P¡ , Vị , and T; , respectively. In terms of R d T¡ , find the total energy entering the system by heat per cycle, the total energy leaving the system by heat per cycle, the efficiency of an engine operating in this cycle, and the efficiency of an engine operating in a Carnot cycle Iween the same temperature extremes P В ЗР, Qi Q3 2P; P; D Q4 V V; 2V;
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter18: Heat Engines, Entropy, And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 61P
Related questions
Question
100%
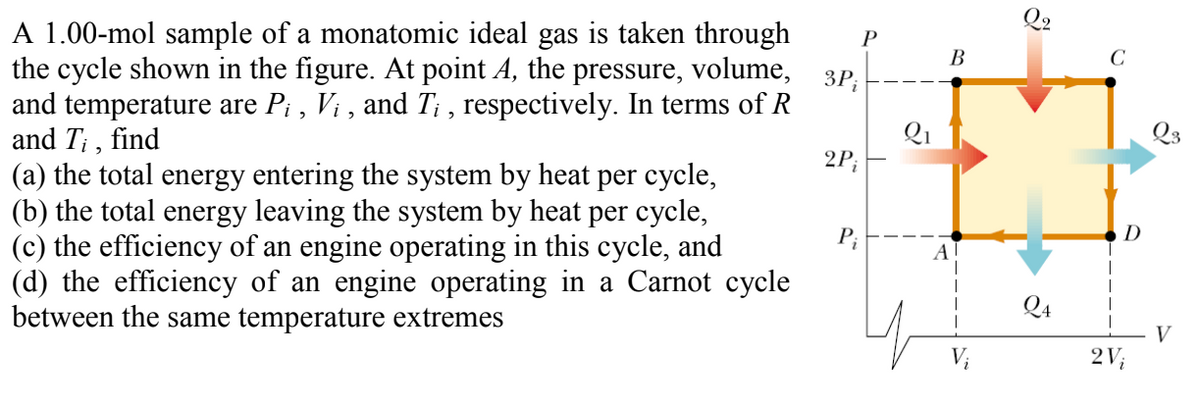
Transcribed Image Text:A 1.00-mol sample of a monatomic ideal gas is taken through
the cycle shown in the figure. At point A, the pressure, volume,
and temperature are P; , Vi , and T; , respectively. In terms of R
and T; , find
(a) the total energy entering the system by heat per cycle,
(b) the total energy leaving the system by heat per cycle,
(c) the efficiency of an engine operating in this cycle, and
(d) the efficiency of an engine operating in a Carnot cycle
between the same temperature extremes
В
C
3P;
Qi
Q3
2P;
P;
Q4
V
V;
2V;
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning