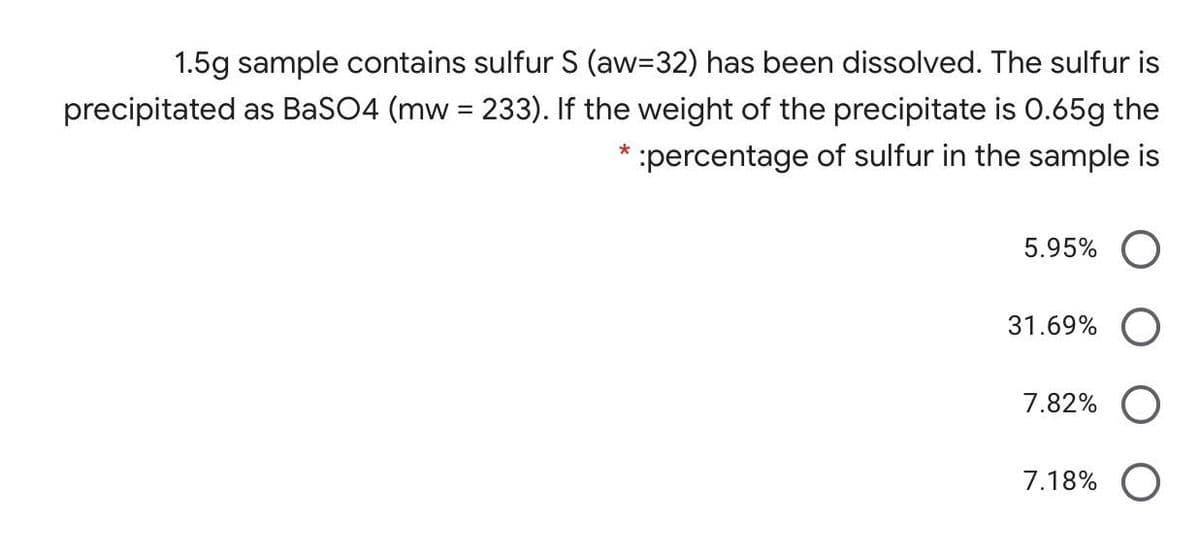
1.5g sample contains sulfur S (aw=32) has been dissolved. The sulfur is precipitated as BaSO4 (mw = 233). If the weight of the precipitate is 0.65g the percentage of sulfur in the sample is 5.95% 31.69% O 7.82% O 7.18% O
Q: 4:[a] A mixture containing only KCI and NaBr is analyzed by the Mohr method. A 0.3172-g sample is…
A:
Q: 3. The mercury in a 1.0023-g soil sample was precipitated with an excess of paraperiodic acid,…
A: Given: Mass of Hg5(IO6)2 = 0.4996 g. And mass of sample used = 1.0023 g.
Q: The arsenic in a 1.223-g sample of a pesticide was converted to H3AsO4 by suitable treatment. The…
A:
Q: Method of separation/chemical reaction (with the brief process) of 3 KG's OF SODIUM BORATE AND 1.5…
A: This invention relates to a process for the production of boric acid from sodium biborate using…
Q: One litre of a saturated aqueous solution of Ag2SO4 (MW = 311.79 g mol- 1) at 25 °C is evaporated to…
A:
Q: A (3.6500 g) of impure ammonium aluminium sulfate (NH4AI(SO4)2)) was treated with ammonia (NH3(aq))…
A: Given: Mass of sample = 3.6500 g. Mass of Al2O3 formed = 0.4935 g.
Q: The arsenic in a 1.010 g sample of a pesticide was converted to H3AsO4 by suitable treatment. The…
A: step: 1 of 3 We have to calculate the percentage of in 1.010 g pesticide. Reaction of arsenic…
Q: The weight of 5 ml of vinegar sample was 5.228 g, was required 30.5 ml of 0.2 M sodium hydroxide…
A: Acid reacts with base to form salt and water and this reaction is known as neutralization reaction.…
Q: Sodium carbonate is recovered as decahydrate from 1000kg of 5% solution of sodium carbonate in…
A: 1.
Q: The arsenic in a 1.010 g sample of a pesticide was converted to H3ASO4 by suitable treatment. The…
A:
Q: mixture containing only KCl and NaBr is analyzed by the Mohr Method. A 0.3172-g sample is dissolved…
A:
Q: The arsenic in a 1.223-g sample of a pesticide was converted to H3ASO4 by suitable treatment. The…
A: Mass of sample = 1.223 g Initial Molarity of AgNO3 = 0.07891 M Initial Volume of AgNO3 = 40 mL or…
Q: A 516.7-mg sample containing a mixture of K,SO, and (NH,),SO, was dissolved in water and treated…
A: Weight of sample = 516.7 mg
Q: 6. The hydrogen sulfide in an 80.0-g sample of crude petroleum was removed by distillation and…
A: 6. Answer - The balanced chemical equation for the problem is: CdSO₄ + H₂S → CdS + H₂SO₄ Getting…
Q: .1724g of a mineral containing MnO₂ was dissolved and then treated with excess iodide according to…
A: A question based on mole concept that is to be accomplished.
Q: Explain the feature of homogeneous products I need only two features !
A: Homogeneous products are the products which consists of particles having same size, quality, color,…
Q: A student investigated the stoichiometry of the reaction of zinc (Zn) with HCI solution and reported…
A:
Q: The arsenic in a 1.010 g sample of a pesticide was converted to H3AsO4 by suitable treatment. The…
A: molarity is defined as the moles of solute upon the volume of sample molarity =molesvolume
Q: D. None of the above 11. A 315mL of water was added to 2100 mL of 19M NaCI solution. What is the new…
A:
Q: and in 4) To adjust the thiosulfate (S2O32) solution to be used as a titrant in the copper…
A:
Q: Calcium in a sample solution is determined by atomic absorption spectroscopy (AAS). A stock solution…
A: According to Beer's law, absorbance of a solution is directly proportional to its concentration.…
Q: 4- Mo in a 0.2711 g sample was precipitated giving 1.1682 g of (NH4)2PO4.12 MoO; . Find percentage…
A: Amount of Mo that was precipitated = 1.1682g Molar mass of precipitates so formed i.e.…
Q: 1.5g sample contains sulfur S (aw=32) has been dissolved. The sulfur is precipitated as BaSO4 (mw =…
A: Given information: Mass of sample = 1.5 g Mass of precipitate = 0.65 g
Q: Potassium dichromate has several industrial applications. To determine the purity of the salt that…
A: KI or I-(aq) reduces potassium dichromate, K2Cr2O7 to Cr3+(aq) and itself gets oxidized to I2. The…
Q: Suppose you have a soil that is made up of 10% organic matter with CEC = 200 cmol/kg, 40% kaolinite…
A: Given: A soil with- 10% organic matter with CEC = 200 cmol/kg 40% kaolinite with CEC = 10 cmol/kg…
Q: 6. A stock solution of iron was prepared by dissolving 1.452g FeCl3 in water, reacting with…
A: Given: Preparation of stock solution 1.452 g FeCl3 in water, reacting with thiocyanate to form…
Q: 2. The solubility of Fe(OH)2is 3.00 x 10-3g in 2.00 liters at 18ºC. What is its Ksp at 18°C? (MW of…
A:
Q: For titration for determination of Chloride, 0.05 AgNO3 and NaCl with a 5% (w/v) K2CrO4 indicator,…
A: In the determination of chloride ion concentration by Mohr's method, NaCl is used as the primary…
Q: A student investigated the stoichiometry of the reaction of zinc (Zn) with HCI solution and reported…
A: Given, mass of Zinc = 0.2158 g volume of HCl = 10 mL concentration of HCl = 1.00 M mass of water…
Q: A 1.250 g sample of bauxite (the primary ore of aluminum) was analyzed for aluminum. The sample was…
A: Given: Weight of sample of bauxite = 1.250 g Mass of Al2O3 recovered =0.2890 g Mass of Ash = 0.0007…
Q: 4. IH a gravimetric determination of sulfur as BaSO4, 0.8863 g of the ignited precipitate is found…
A: This problem requires basic analytical approach.
Q: Calculate the molar concentration of H3PO4 commercial reagent that is 86% (w/w) and has a specific…
A:
Q: A water containing 50 mg/L of phosphate (PO.) is flowing in an open channel at a volumetric flow…
A: One liter of water contains 50mg of phosphate ions and the total liter of water flowing per day is…
Q: The solubility of a compound (X2S) is 1.3 x 10–4 M at 298K. Ksp for this compound is: ]1.7 × 10-8…
A: Hii there, since there are multiple questions posted. We are answering first question. If you need…
Q: f to a solution of NaOH, in water and ethanol (20 ° C) 3 mmol of A (106.11 g / mol) and 2 mmol of B…
A: Given reaction is 2A + B ⟶ C It is given that: Amount of A = 3 mmol Amount of B = 2 mmol Amount of…
Q: The arsenic in a 1.223-g sample of a pesticide was converted to H3AsO4 by suitable treatment. The…
A:
Q: To prepare orange chrome dissolve 2g of lead acetate in 25cc of Warm distilled water, add 4cc oF 2…
A: Chrome orange is a mixed oxide with the chemical formula, Pb2CrO5.
Q: Gravimetric analysis of Fe3O4 (MW = 232 g/mole) may be undertaken with the following reactions:…
A:
Q: The sulfur content of an iron pyrite ore sample is determined by converting it to H2S gas, absorbing…
A: H2S + I2 ----> S + 2I- + 2H+ I2 + 2S2O3-2 ----> 2I- + S4O6-2 Valency factor of H2S = 2 (O.S…
Q: he sulfate in 266.5 mg sample was precipitated as BaSO4 by addition of 27.00 mL of 0.0340 M BaCl2.…
A:
Q: A student investigated the stoichiometry of the reaction of zinc (Zn) with HCI solution and reported…
A:
Q: A scientist was tasked to extract Fe from an aqueous suspension that contains 106 g of Fe2O3. The…
A: Given: mass of Fe2O3 = 106 g Molar mass of Fe2O3 = 159.69 g/mol
Q: A scientist was tasked to extract Fe from an aqueous suspension that contains 106 g of Fe2O3. The…
A: Given: mass of Fe2O3 = 106 g Molar mass of Fe2O3 = 159.69 g/mol Molar mass of Fe = 55.845 g/mol
Q: Determine the solubility for a saturated solution of Tl2S. Ksp = 6.0 x 10-22
A: Solubility Product is the dynamic equilibrium that exists when a chemical compound in solid state is…
Q: The concentration of CO in the air can be determined by passing a known volume of air through a tube…
A: Parts per million is a commonly used unit of concentration for small values and parts per million…
Q: An analyst was assigned to work a sample with minerals. This iron-containing sample was analyzed by…
A: First , we calculate moles of ferric oxide by dividing mass with molar mass. based on reaction…
Q: he mercury in a 0.8142-g sample was precipitated with an excess of paraperiodic acid, H5IO6:…
A: The ratio of mass of the particular compound to the total mass multiply by 100 is known as percent…
Q: om the following data calculate the normalities of the acid and base solutions: weight of pure…
A: Here, Nax 0.982= Nbx 1ml Acid as, 0.2448105.98 =Va ×Na2.31×10-3 =Va ×Na Then, 0.84Nb =43.65-VaNa
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- A 0.4852g sample of an iron ore was dissolved in acid to the 3+ state, then precipitated as Fe2O3 x H2O. The pp was filtered, washed and calcined to Fe2O3, which gave a weight of 0.2481g. Calculate the Fe % in the sample.One litre of a saturated aqueous solution of Ag2SO4 (MW = 311.79 g mol- 1) at 25 °C is evaporated to dryness. 4.844 g of Ag2SO4 residue was produced. What is the solubility product (Ksp)?An ore containing Fe3O4 was analyzed by dissolving a 1.5419-g sample in concentrated HCl. HNO3 was then added to oxidize any Fe2+ to Fe3+. The Fe3+ was precipitated as Fe(OH)3 by adding NH3. The precipitate was collected by filtration and was finally heated yielding 0.8525 g of Fe2O3. Report the concentration of Fe3O4 in the sample as %(w/w). (Note: the final answer is said to be 53.4%)
- The mercury in a 0.8142-g sample was precipitated with an excess of paraperiodic acid, H5IO6: 5 Hg 2+ + 2 H5IO6 ---> Hg5(IO6)2 (s) + 10 H + The precipitate (MW = 1448.8 g/mol) was filtered, washed free of precipitating agent, dried, and weighed, and 0.4114 g was recovered. Calculate the a) % Hg (200.6 g/mol) b) % Hg2Cl2 (472.1 g/mol)An analyst was assigned to work a sample with minerals. This iron-containing sample was analyzed by dissolving a 1.3142g sample in concentrated HCl. The resulting solution was diluted with water and iron (III) was precipitated as the hydrated oxide Fe2O3 xH2O by the addition of HN3. After filtration and washing, the residue was calcined at high temperature to produce 0.5488g of pure Fe2O3 (159.69g / mol). Determine the following:a) the% Fe (55.847 g / mol) and the% Fe3O4 (231.54 g / mol) in the sample.The concentration of CO in the air can be determined by passing a known volume of air through a tube containing I2O5, resulting in the formation of CO2 and I2. I2 is removed from the tube by distillation and collected in a solution containing excess KI, producing I3-. I3- is titrated with a standard solution of Na2S2O3. A 4.79 L air sample was sampled as described here, requiring 7.17 ml of 0.00329 M Na2S2O3 to reach the endpoint in a typical analysis. If the density of the air is 1.23×10^-3 g/ml, what is the amount of CO in the air in ppm? (CO: 28 g/ml)
- Mo in a 0.2711g sample was precipitated giving 1.1682g of (NH4)2PO4.12 MoO3. Find the percentage Mo , P (at wt = 30.97), N=14,Mo=95.9,H=1,O=16A 1.1324 g sample of magnetite ore was dissolved in concentrated HCl to give a solution that contained a mixture of Fe2+ and Fe3+. Nitric acid was added and the solution was boiled for a few minutes, which converted all of the iron to Fe3+. The Fe3+ was then precipitated as Fe2O3*xH2O by addition of NH3. After filtration and washing, the residue was ignited at a high temp to give 0.5394 g of pure Fe2O3. What is the percent Fe and the percent Fe3O4 in the sample?Potassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.07
- Gravimetric analysis of Fe3O4 (MW = 232 g/mole) may be undertaken with the following reactions: Fe3O4 → Fe2O3 → Fe (OH)3. Weight of sample containing 8.00% Fe3O4 that must be taken to obtain a precipitate of Fe(OH)3 (MW = 107 g/mole) that weighs 150 mg is . a. 0.108 g b. 0.325 g c. 1.355 g d. 4.065 g Amount of Fe2O3 (MW = 160 g/mole) from which 150 mg of Fe(OH)3 (MW = 107 g/mole) may be obtained is . a. 0.112 g b. 0.224 g c. 0.448 g d. none of the other choicesAn impure sample of Na3PO3 weighing 0.1 g is dissolved in 35 mL of water. A solution containing 45 mL of 3% w/v HgCl2, 30 mL of 10% w/v sodium acetate, and 10 mL of glacial acetic acid is then prepared. After digesting, filtering, and rinsing the precipitate, 0.2857 g of Hg2Cl2 is obtained. Report the purity of the original sample as % w/w Na3PO3.A (3.6500 g) of impure ammonium aluminium sulfate (NH4Al(SO4)2)) was treated with ammonia (NH3(aq)) producing hydrous alumina Al2O3.xH2O. The collected precipitate was filtered, washed, and ignited at 1000 °C to give 0.4935 g Al2O3 (Mw 101.9635 g/mol). Determine: a. % Al2O3 b. %Al c. Express concentration of Al in ppm.

