Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.12: Over The Hill: Reversing Reactions
Problem 5E
Related questions
Question
Transcribed Image Text:B
the intermediate is not present in the energy diagram
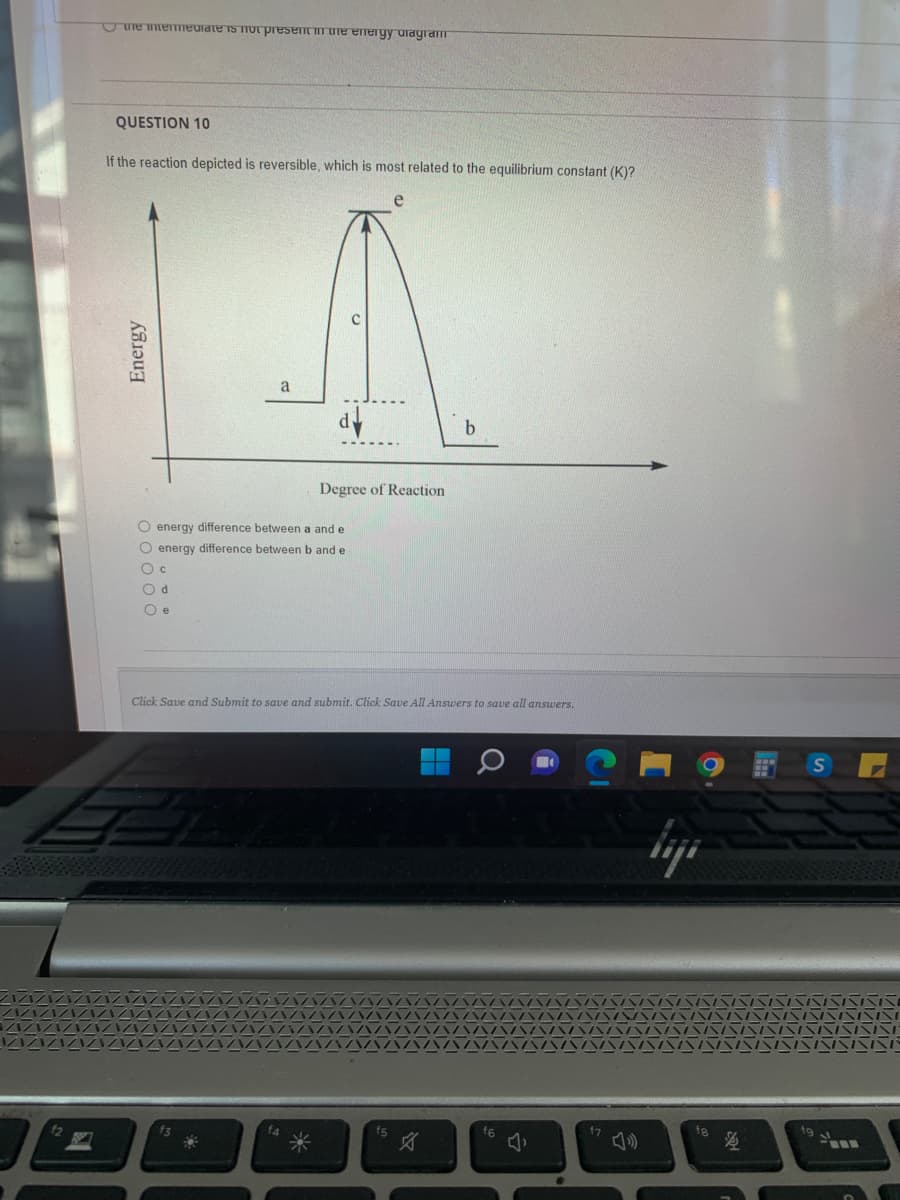
QUESTION 10
If the reaction depicted is reversible, which is most related to the equilibrium constant (K)?
Energy
a
f3
d
O energy difference between a and e
O energy difference between b and e
O c
Od
Oe
*
Degree of Reaction
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
f5
b
H
a
f6
3
fg
$
Transcribed Image Text:Question Completion Status:
W
O energy difference between b and e
Od
Oe
QUESTION 11
When a reversible reaction has reached an equilibrium:
O All the provided statements regarding equilibrium are true
O the concentration of reactants = the concentration of products
O both the forward reaction and the reverse reaction have stopped
the forward reaction and the reverse reaction are occuring at the same rate
O the forward reaction has stopped
QUESTION 12
If the following exothermic reaction is at equilibrium, what kind of changes will drive the reaction further to the right (i.e. make more product)? Follow Le Chatelier's Principle.
N2(g) + 3 H₂ (9) <-> 2 NH3 (9)
O increasing temperature (put in more heat)
O remove some H₂
O add extra ammonia (NH3)
O increasing pressure
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
f3
#3
22
F
f4
*
54
$
R
f5
%
5
16
T
6
f7
Y
lyi
&
7
18
$
7
U
* 00
19
8
4
S
8
f10:
num Ik
9
5
f
(1₂³)
O
O
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning