10. At a temperature of 30 °C, the pressure of the gas in a deodorant can is 3 atm. Calculate the pressure (Pag)of the gas when it is heated to 1440 R.
10. At a temperature of 30 °C, the pressure of the gas in a deodorant can is 3 atm. Calculate the pressure (Pag)of the gas when it is heated to 1440 R.
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter45: Domestic Refrigerators And Freezers
Section: Chapter Questions
Problem 8RQ: Frost accumulates on the evaporators of forced-draft refrigerators because A. they are generally...
Related questions
Question
10. At a temperature of 30 °C, the pressure of the gas in a deodorant can is 3 atm.
Calculate the pressure (Pag)of the gas when it is heated to 1440 R.
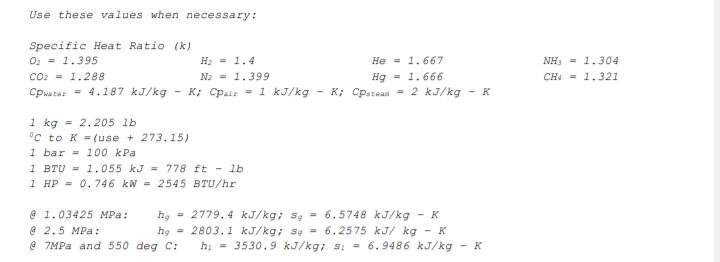
Transcribed Image Text:Use these values when necessary:
Specific Heat Ratio (k)
O2 - 1.395
Co2 = 1.288
Cpwater = 4.187 kJ/kg
H2 - 1.4
He = 1.667
NH3 = 1.304
N2 = 1.399
Hg = 1.666
CH: = 1.321
K; Cpair = 1 kJ/kg
- K; Cpatean = 2 kJ/kg - K
1 kg - 2.205 lb
"C to K = (use + 273.15)
1 bar = 100 kPa
1 BTU = 1.055 kJ = 778 ft - lb
1 HP = 0. 746 kW = 2545 BTU/hr
= 6.5748 kJ/kg - K
@ 1.03425 MPa:
@ 2.5 MPa :
@ 7MPA and 550 deg C:
= 2779. 4 kJ/kg; Sg
hg = 2803.1 kJ/kg; Sg = 6.2575 kJ/ kg
h: = 3530.9 kJ/kg; s: = 6.9486 kJ/kg
- K
- K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning