1 Introduction To Chemistry 2 Atoms And Molecules 3 Molecules, Moles, And Chemical Equations 4 Stoichiometry 5 Gases 6 The Periodic Table And Atomic Structure 7 Chemical Bonding And Molecular Structure 8 Molecules And Materials 9 Energy And Chemistry 10 Entropy And The Second Law Of Thermodynamics 11 Chemical Kinetics 12 Chemical Equilibrium 13 Electrochemistry 14 Nuclear Chemistry Chapter10: Entropy And The Second Law Of Thermodynamics
Chapter Questions Section: Chapter Questions
Problem 1CO Problem 2CO: . explain the concept of entropy in your own words. Problem 3CO Problem 4CO: . state the second law of thermodynamics in words and equations and use it to predict spontaneity. Problem 5CO Problem 6CO Problem 7CO Problem 8CO Problem 9CO Problem 10CO Problem 10.1PAE Problem 10.2PAE Problem 10.3PAE Problem 10.4PAE Problem 10.5PAE Problem 10.6PAE: Use the web to learn how many pounds of plastics are re cycled in your area each year. How has this... Problem 10.7PAE: On the basis of your experience, predict which of the following reactions are spontaneous. (a)... Problem 10.8PAE: In the thermodynamic definition of a spontaneous process, why is it important that the phrase... Problem 10.9PAE: 1f the combustion of butane is spontaneous, how can you carry a butane lighter safely in your pocket... Problem 10.10PAE: Identify each of the processes listed as spontaneous or nons-pontaneous. For each nonspontaneous... Problem 10.11PAE: Identify each of the processes listed as spontaneous or non-spontaneous. For each non spontaneous... Problem 10.12PAE: Athletic trainers use instant ice packs that can be cooled quickly on demand. Squeezing the pact... Problem 10.13PAE: Are any of the following exothermic processes not spontaneous under any circumstances? (a) Snow... Problem 10.14PAE: Enthalpy changes often help predict whether or not a process will be spontaneous. What type of... Problem 10.15PAE: When a fossil fuel burns, is that fossil fuel the system? Explain your answer. Problem 10.16PAE: Murphy's law is a whimsical rule that says that anything that can go wrong will go wrong. But in an... Problem 10.17PAE Problem 10.18PAE: Some games include dice with more than six sides. If you roll two eight-sided dice, with faces... Problem 10.19PAE: How does probability relate to spontaneity? Problem 10.20PAE Problem 10.21PAE: For each pair of items, tell which has the higher entropy and explain why. (a) Item 1, a sample of... Problem 10.22PAE: For each process, tell whether the entropy change of the system is positive or negative, (a) A... Problem 10.23PAE: Without doing a calculation, predict whether the entropy change will be positive or negative when... Problem 10.24PAE: For the following chemical reactions, predict the sign of S for the system. (Note that this should... Problem 10.25PAE: What happens to the entropy of the universe during a spontaneous process? Problem 10.26PAE Problem 10.27PAE: One statement of the second law of thermodynamics is that heat cannot be turned completely into... Problem 10.28PAE Problem 10.29PAE Problem 10.30PAE: Which reaction occurs with the greater increase in entropy? Explain your reasoning. (a)... Problem 10.31PAE: Which reaction occurs with the greater increase in entropy? Explain your reasoning. (a)... Problem 10.32PAE: Methanol is burned as fuel in some race cars. This makes it clear that the reaction is spontaneous... Problem 10.33PAE: Limestone is predominantly CaCO3, which can undergo the reaction CaCO3(s)CaO(s)+CO2(g). We know from... Problem 10.34PAE: Suppose that you find out that a system has an absolute entropy of zero. What else can you conclude... Problem 10.35PAE: Use tabulated thermodynamic data to calculate the standard entropy change of each of the reactions... Problem 10.36PAE Problem 10.37PAE: Calculate S for the dissolution of magnesium chloride: MgCl2(s)Mg2+(aq)+2Cl(aq) . Use your... Problem 10.38PAE: Calculate the standard entropy change for the reaction CO2(g)+2H2O(l)CH4(g)+2O2(g) . What does the... Problem 10.39PAE: Through photosynthesis, plants build molecules of sugar containing several carbon atoms from carbon... Problem 10.40PAE: Find websites describing two different attempts to reach the coldest temperature on record. What... Problem 10.41PAE Problem 10.42PAE Problem 10.43PAE: Under what conditions does G allow us to predict whether a process is spontaneous? Problem 10.44PAE: There is another free energy state function, the Helmholtz free energy (A), defined as A = E — TS.... Problem 10.45PAE: 10.45 Calculate G at 45°C for reactions for which (a) H = 293 kJ; S= -695 J/K (b) H= -1137 kJ; S =... Problem 10.46PAE: 10.46 Discuss the effect of temperature change on the spontaneity of the following reactions at 1... Problem 10.47PAE: The reaction CO2(g)+H2(g)CO(g)+H2O(g) is not spontaneous at room temperature but becomes spontaneous... Problem 10.48PAE Problem 10.49PAE Problem 10.50PAE: For the reaction NO(g)+NO2(g)N2O3(g) , use tabulated thermodynamic data to calculate H and S. Then... Problem 10.51PAE: 10.51 The combustion of acetylene was used in welder's torches for many years because it produces a... Problem 10.52PAE: Natural gas (methane) is being used in experimental vehicles as a clean-burning fuel. (a) Write the... Problem 10.53PAE: Silicon forms a series of compounds analogous to the al-kanes and having the general formula... Problem 10.54PAE: Explain why Gf of O2 (g) is zero. Problem 10.55PAE: Using tabulated thermodynamic data, calculate G for these reactions. (a) Fe(s)+2HCl(g)FeCl2(s)+H2(g)... Problem 10.56PAE: Using tabulated thermodynamic data, calculate G for these reactions. (a)... Problem 10.57PAE: Calculate G for the dissolution of both sodium chloride and silver chloride using data from Appendix... Problem 10.58PAE: Phosphorus exists in multiple solid phases, including two known as red phosphorus and white... Problem 10.59PAE: 10.59 The normal melting point of benzene, C6H6, is 5.5°C. For the process of melting solid benzene,... Problem 10.60PAE Problem 10.61PAE: Estimate the temperature range over which each of the following reactions is spontaneous. (a)... Problem 10.62PAE: Recall that incomplete combustion of fossil fuels occurs when too little oxygen is present and... Problem 10.63PAE: During polymerization, the system usually becomes more ordered as monomers link together. Could an... Problem 10.64PAE Problem 10.65PAE Problem 10.66PAE: The recycling of polymers represents only one industrial process that allows creating order in one... Problem 10.67PAE Problem 10.68PAE: When ice melts, its volume decreases. Despite this fact, the entropy of the system increases.... Problem 10.69PAE: 10.69 If a sample of air were separated into nitrogen and oxygen molecules (ignoring other gases... Problem 10.70PAE Problem 10.71PAE: An explosion brings down an old building, leaving behind a pile of rubble. Does this cause a... Problem 10.72PAE Problem 10.73PAE Problem 10.74PAE Problem 10.75PAE Problem 10.76PAE: Some say that the job of an engineer is to fight nature and the tendencies of entropy. (a) Does this... Problem 10.77PAE: A beaker of water at 40C (on the left in the drawing) and a beaker of ice water at 0°C are placed... Problem 10.78PAE: Why is it usually easier to use G to determine the spontaneity of a process rather than Su ? Problem 10.79PAE: The molecular scale pictures below show snapshots of a strong acid at three different instants after... Problem 10.80PAE Problem 10.81PAE: Diethyl ether is a liquid at normal temperature and pressure, and it boils at 35 C. Given that H is... Problem 10.82PAE: Calculate the entropy change, S , for the vaporization of ethanol, C2H5OH, at the boiling point of... Problem 10.83PAE: Gallium metal has a melting point of 29.8°C. Use the information below to estimate the boiling point... Problem 10.84PAE: Methane can be produced from CO and H2.The process might be done in two steps, as shown below, with... Problem 10.85PAE: 10.85 Iodine is not very soluble in water, but it dissolves readily in a solution containing iodide... Problem 10.86PAE: The enthalpy of vaporization for water is 40.65 kJ mol-1. As a design engineer for a project in a... Problem 10.87PAE: Determine whether each of the following statements is true or false. If false, modify to make the... Problem 10.88PAE: Nickel metal reacts with carbon monoxide to form tetra-carbonyl nickel, Ni(CO)4:... Problem 10.89PAE: Polyethylene has a heat capacity of 2,3027 J g-1 °C-1. You need to decide if 1.0 ounce of... Problem 10.90PAE: A key component in many chemical engineering designs is the separation of mixtures of chemicals. (a)... Problem 10.91PAE: The reaction shown below is involved in the refining of iron. (The table that follows provides all... Problem 10.92PAE: Using only the data given below, determine G for the following reaction: NO(g)+O(g)NO2(g) (Remember... Problem 10.93PAE: The graph below shows G as a function of temperature for the synthesis of ammonia from nitrogen and... Problem 10.94PAE Problem 10.95PAE Problem 10.96PAE Problem 10.97PAE Problem 10.98PAE Problem 10.99PAE: Thermodynamics provides a way to interpret everyday occurrences. If you live in northern climates,... Problem 10.100PAE Problem 10.101PAE: 10.101 Fluorine reacts with liquid water to form gaseous hydrogen fluoride and oxygen. (a) Write a... Problem 10.102PAE: 10.102 Ammonia can react with oxygen gas to form nitrogen dioxide and water. (a) Write a balanced... Problem 10.103PAE Problem 10.104PAE: 10.104 (a) When a chemical bond forms, what happens to the entropy of the system? (b)... Problem 10.91PAE: The reaction shown below is involved in the refining of iron. (The table that follows provides all...
Related questions
Please answer and show all work for parts A-C, thank you!! Thermodynamics table is shown below :)
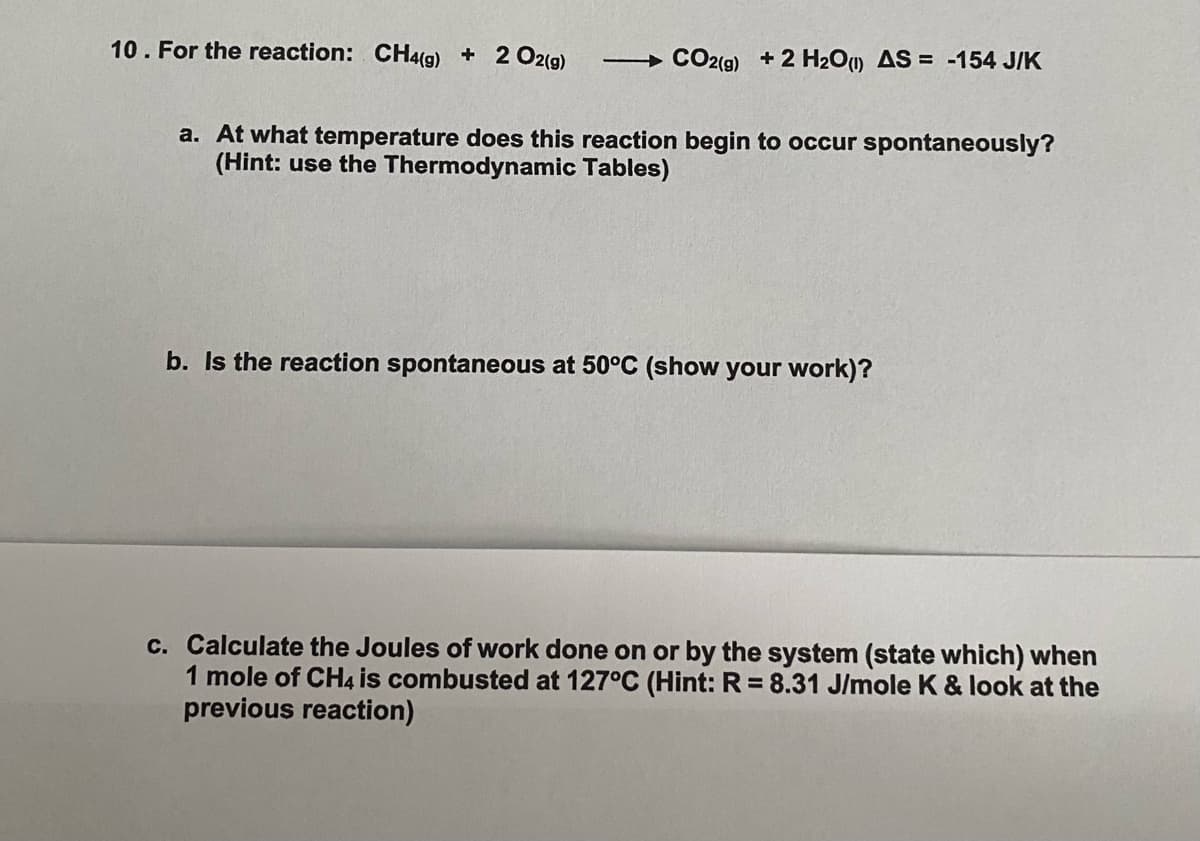
Transcribed Image Text: 10. For the reaction: CH4(g) + 2 O2(g)
CO2(9) + 2 H20) AS = -154 J/K
a. At what temperature does this reaction begin to occur spontaneously?
(Hint: use the Thermodynamic Tables)
b. Is the reaction spontaneous at 50°C (show your work)?
c. Calculate the Joules of work done on or by the system (state which) when
1 mole of CH4 is combusted at 127°C (Hint: R = 8.31 J/mole K & look at the
previous reaction)
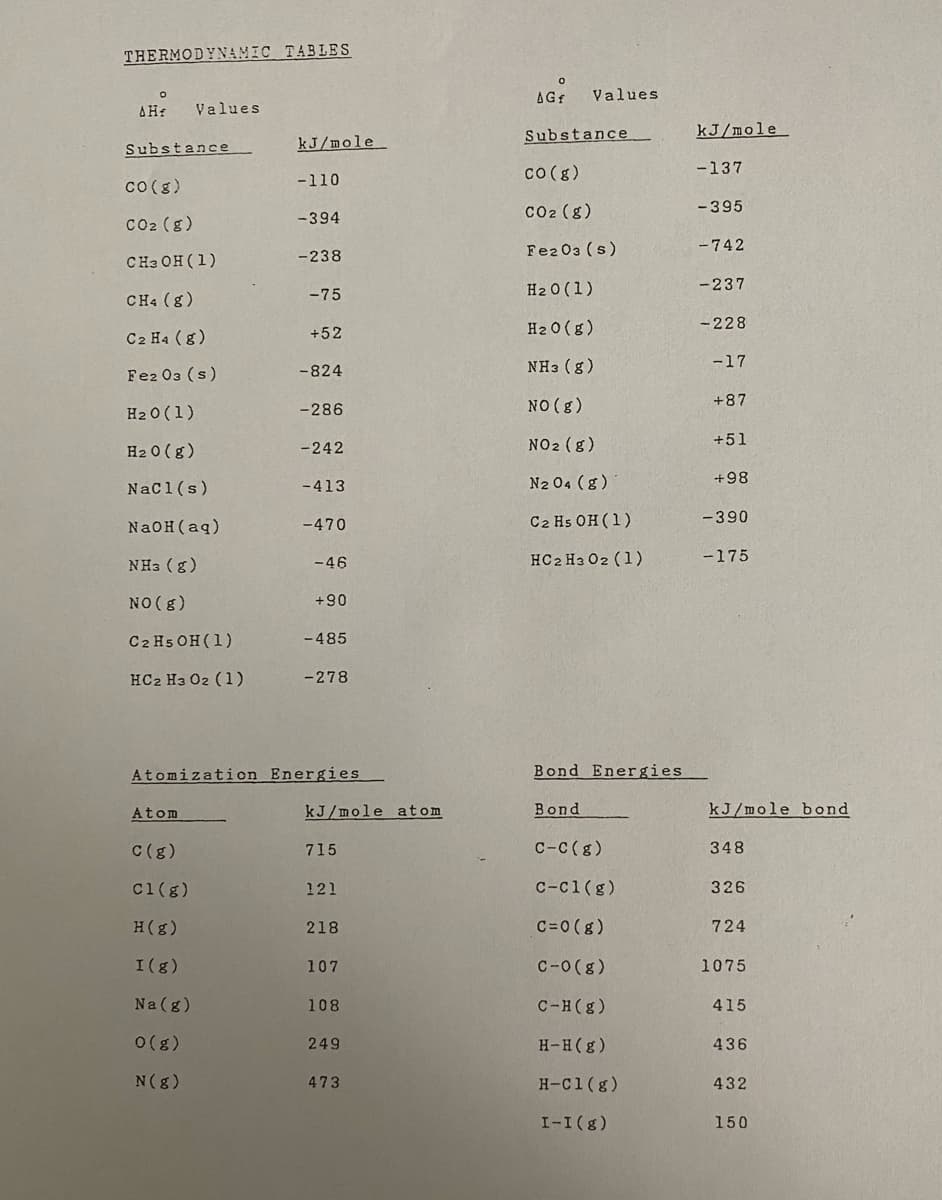
Transcribed Image Text: THERMODYNAMIC TABLES
AGf
Values
ΔΗ
Values
Substance
kJ/mole
Substance
kJ/mole
co (g)
-137
Co(g)
-110
Co2 (g)
-395
Co2 (g)
-394
Fe2 03 (s)
-742
CHa OH (1)
-238
H2 0 (1)
-237
CH4 (g)
-75
H2 0 (g)
- 228
C2 Ha (g)
+52
NH3 (g)
-17
Fe2 03 (s)
-824
NO (g)
+87
H2 0 (1)
-286
-242
NO2 (g)
+51
H2 0 (g)
Nac1(s)
N2 04 (g)
+98
-413
-470
С2 Hs ОН (1)
-390
NAOH (aq)
NH3 (g)
HC2 H3 02 (1)
-175
-46
NO (g)
+90
C2 Hs OH (1)
- 485
HC2 H3 02 (1)
-278
Atomization Energies
Bond Energies
Atom
kJ/mole atom
Bond
kJ/mole bond
C(g)
715
C-C(g)
348
c1(g)
121
c-c1(g)
326
H(g)
218
C=0 (g)
724
I(g)
107
C-0(g)
1075
Na(g)
108
C-H(g)
415
0(g)
249
H-H(g)
436
N(8)
473
H-C1(g)
432
I-I(g)
150
Science that deals with the amount of energy transferred from one equilibrium state to another equilibrium state.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images