10. Which of the following is not part of the collision theory? a. b. C. d. e. a chemical system consists of particles that are in constant motion a reaction must involve collisions of particles an effective collision has sufficient energy and correct orientation an ineffective collision can still cause a reaction the rate of the reaction depends on the frequency of effective collisions
10. Which of the following is not part of the collision theory? a. b. C. d. e. a chemical system consists of particles that are in constant motion a reaction must involve collisions of particles an effective collision has sufficient energy and correct orientation an ineffective collision can still cause a reaction the rate of the reaction depends on the frequency of effective collisions
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Hey stuck on this sheet :)), I dont need an explanation or diagram :)
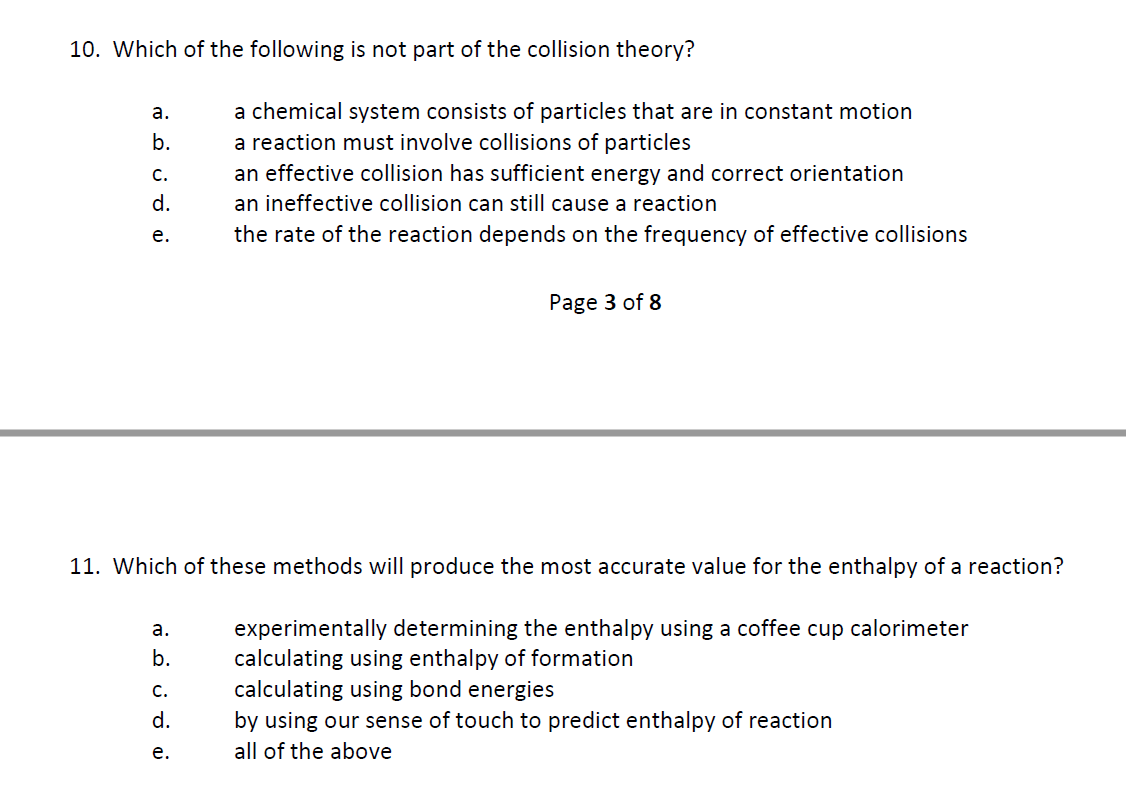
Transcribed Image Text:10. Which of the following is not part of the collision theory?
a.
b.
C.
d.
e.
a.
b.
a chemical system consists of particles that are in constant motion
a reaction must involve collisions of particles
an effective collision has sufficient energy and correct orientation
an ineffective collision can still cause a reaction
the rate of the reaction depends on the frequency of effective collisions
11. Which of these methods will produce the most accurate value for the enthalpy of a reaction?
experimentally determining the enthalpy using a coffee cup calorimeter
calculating using enthalpy of formation
calculating using bond energies
by using our sense of touch to predict enthalpy of reaction
all of the above
C.
d.
e.
Page 3 of 8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning