10.28 Given below are values of GE /J.mol-¹, HE /J.mol-¹, and CE/J.mol-¹.K-¹ for some equimolar binary liquid mixtures at 298.15 K. Estimate values of GE, HE, and SE at 328.15 K for one of the equimolar mixtures by two procedures: (I) Use all the data; (II) Assume CE = 0. Compare and discuss your results for the two procedures. (a) Acetone/chloroform: GE = −622, HE = − 1920, CE = 4.2. == (b) Acetone/n-hexane: GE = 1095, HE = 1595, CF = 3.3. (c) Benzene/isooctane: (d) Chloroform/ethanol: GE = 407, HE = 984, CE = -2.7. GE = 632, HE = -208, CE = 23.0. (e) Ethanol/n-heptane: GE = 1445, HE = 605, C = 11.0. (f) Ethanol/water: G¹ = 734, HE = −416, C² = 11.0. (g) Ethyl acetate/n-heptane: GE = 759, H² = 1465, CE = −8.0.
10.28 Given below are values of GE /J.mol-¹, HE /J.mol-¹, and CE/J.mol-¹.K-¹ for some equimolar binary liquid mixtures at 298.15 K. Estimate values of GE, HE, and SE at 328.15 K for one of the equimolar mixtures by two procedures: (I) Use all the data; (II) Assume CE = 0. Compare and discuss your results for the two procedures. (a) Acetone/chloroform: GE = −622, HE = − 1920, CE = 4.2. == (b) Acetone/n-hexane: GE = 1095, HE = 1595, CF = 3.3. (c) Benzene/isooctane: (d) Chloroform/ethanol: GE = 407, HE = 984, CE = -2.7. GE = 632, HE = -208, CE = 23.0. (e) Ethanol/n-heptane: GE = 1445, HE = 605, C = 11.0. (f) Ethanol/water: G¹ = 734, HE = −416, C² = 11.0. (g) Ethyl acetate/n-heptane: GE = 759, H² = 1465, CE = −8.0.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
![[2] Ch 10 # 28, estimate by procedure (1) only](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fae4237e8-0a91-4962-8db1-4f59bb0866b5%2F80836d9c-9dd3-4578-b485-db06d81b31a1%2F3ux6anmg_processed.jpeg&w=3840&q=75)
Transcribed Image Text:[2] Ch 10 # 28, estimate by procedure (1) only
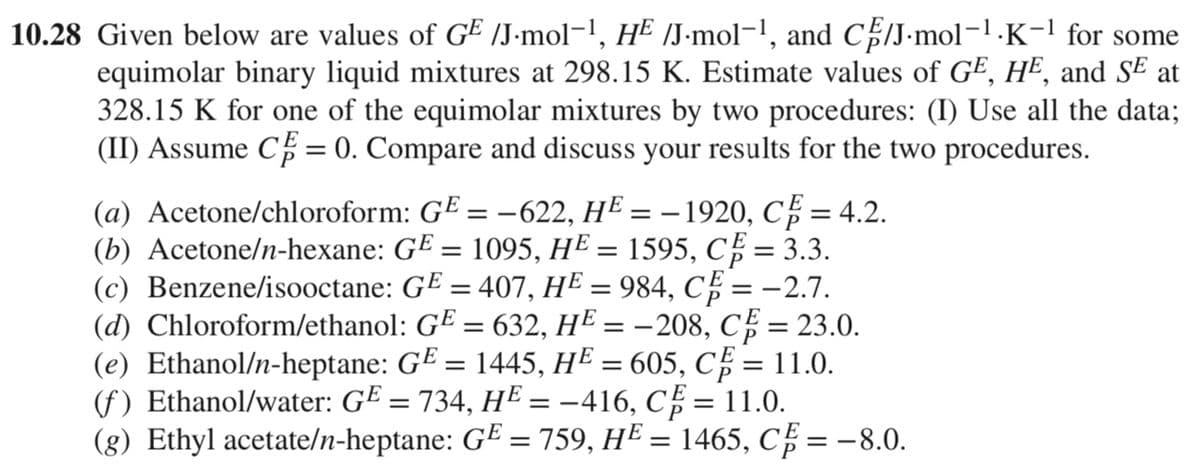
Transcribed Image Text:10.28 Given below are values of GE /J.mol-¹, H² /J.mol-¹, and CE/J.mol-¹.K−¹ for some
equimolar binary liquid mixtures at 298.15 K. Estimate values of GE, HE, and SE at
328.15 K for one of the equimolar mixtures by two procedures: (I) Use all the data;
(II) Assume C = 0. Compare and discuss your results for the two procedures.
(a) Acetone/chloroform: GE = -622, H² = − 1920, CF = 4.2.
(b) Acetone/n-hexane: GE = 1095, HE = 1595, CF = 3.3.
(c) Benzene/isooctane:
(d) Chloroform/ethanol:
GE = 407, HE = 984, CF = -2.7.
G¹ = 632, H² = -208, C = 23.0.
(e) Ethanol/n-heptane: G¤ = 1445, HE = 605, C = 11.0.
(f) Ethanol/water: G² = 734, HE = -416, C = 11.0.
(g) Ethyl acetate/n-heptane: GE = 759, H² = 1465, CF = -8.0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The