10.3 For a linear molecule of polyethylene of molar mass 119,980 g mol-¹, calculate: (a) the contour length of the molecule, (b) the end-to-end distance in the fully-extended molecule, and (c) the root-mean-square end-to-end distance according to the valence angle model. In the calculations, end groups can be neglected and it may be assumed that the C-C bonds are of length 0.154 nm and that the valence angles are 109.5°. Comment upon the values
10.3 For a linear molecule of polyethylene of molar mass 119,980 g mol-¹, calculate: (a) the contour length of the molecule, (b) the end-to-end distance in the fully-extended molecule, and (c) the root-mean-square end-to-end distance according to the valence angle model. In the calculations, end groups can be neglected and it may be assumed that the C-C bonds are of length 0.154 nm and that the valence angles are 109.5°. Comment upon the values
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter7: Bonding In Organic Molecules
Section: Chapter Questions
Problem 29P: Compare the bonding in formic acid (HCOOH) with that in its conjugate base formate ion (HCOO). Each...
Related questions
Question
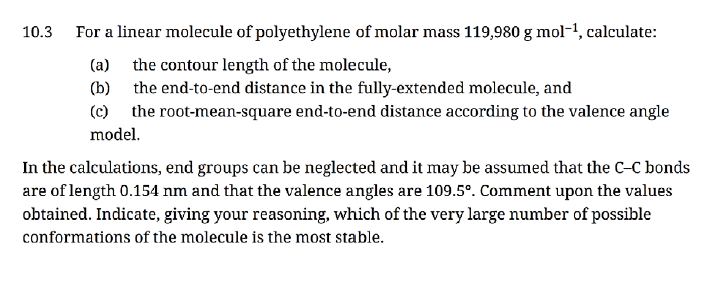
Transcribed Image Text:10.3 For a linear molecule of polyethylene of molar mass 119,980 g mol-¹, calculate:
(a)
the contour length of the molecule,
(b)
the end-to-end distance in the fully-extended molecule, and
(c) the root-mean-square end-to-end distance according to the valence angle
model.
In the calculations, end groups can be neglected and it may be assumed that the C-C bonds
are of length 0.154 nm and that the valence angles are 109.5°. Comment upon the values
obtained. Indicate, giving your reasoning, which of the very large number of possible
conformations of the molecule is the most stable.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning