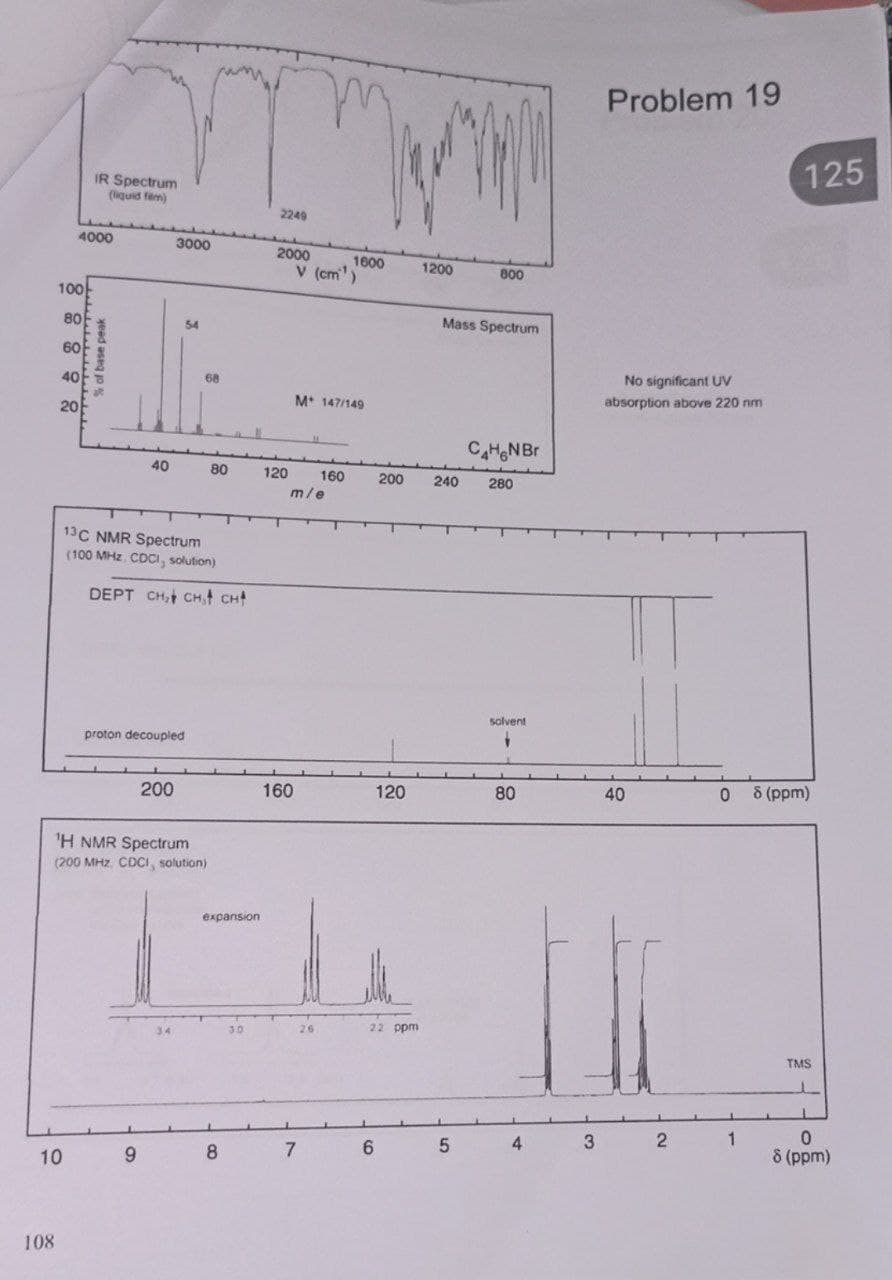
100 4000 10 IR Spectrum (liquid fem) 80 88888 % of base peak 60 3000 54 40 13C NMR Spectrum (100 MHz. CDCI, solution) 9 68 DEPT CH, CH, CH proton decoupled 200 ¹H NMR Spectrum (200 MHz. CDCI, solution) 34 80 expansion 30 8 2249 2000 160 V (cm) 120 160 m/e M 147/149 1600 7 26 200 120 22 ppm 6 ww 1200 800 Mass Spectrum C₂HGN Br 240 280 5 solvent + 80 4 3 Problem 19 No significant UV absorption above 220 nm 0 40 2 1 125 8 (ppm) TMS 1 0 8 (ppm)
Analyzing Infrared Spectra
The electromagnetic radiation or frequency is classified into radio-waves, micro-waves, infrared, visible, ultraviolet, X-rays and gamma rays. The infrared spectra emission refers to the portion between the visible and the microwave areas of electromagnetic spectrum. This spectral area is usually divided into three parts, near infrared (14,290 – 4000 cm-1), mid infrared (4000 – 400 cm-1), and far infrared (700 – 200 cm-1), respectively. The number set is the number of the wave (cm-1).
IR Spectrum Of Cyclohexanone
It is the analysis of the structure of cyclohexaone using IR data interpretation.
IR Spectrum Of Anisole
Interpretation of anisole using IR spectrum obtained from IR analysis.
IR Spectroscopy
Infrared (IR) or vibrational spectroscopy is a method used for analyzing the particle's vibratory transformations. This is one of the very popular spectroscopic approaches employed by inorganic as well as organic laboratories because it is helpful in evaluating and distinguishing the frameworks of the molecules. The infra-red spectroscopy process or procedure is carried out using a tool called an infrared spectrometer to obtain an infrared spectral (or spectrophotometer).
Step by step
Solved in 2 steps with 1 images









