100 microliters (100 µL) of the mixture. Measurements during the first 18 minutes after the mixture reaches 95°C are listed in the following table. Chemical Reaction Time, t (minutes) Activity, r (U/100µL) 0.10 0.10 4. 0.25 6. 0.60 8. 1.00 10 1.40 12 1.55 14 1.75 16 1.90 18 1.95 (a) Find the function for the logistic model that gives the activity of the chemical reaction in U/100uL, where t is the time in minutes after the mixture reaches a temperature of 95°C, with data from 0sts 18. (Round all numerical values to three decimal places.) r(t) = U/100pl What is the limiting value for this logistic function?
100 microliters (100 µL) of the mixture. Measurements during the first 18 minutes after the mixture reaches 95°C are listed in the following table. Chemical Reaction Time, t (minutes) Activity, r (U/100µL) 0.10 0.10 4. 0.25 6. 0.60 8. 1.00 10 1.40 12 1.55 14 1.75 16 1.90 18 1.95 (a) Find the function for the logistic model that gives the activity of the chemical reaction in U/100uL, where t is the time in minutes after the mixture reaches a temperature of 95°C, with data from 0sts 18. (Round all numerical values to three decimal places.) r(t) = U/100pl What is the limiting value for this logistic function?
Algebra & Trigonometry with Analytic Geometry
13th Edition
ISBN:9781133382119
Author:Swokowski
Publisher:Swokowski
Chapter7: Analytic Trigonometry
Section7.3: The Addition And Subtraction Formulas
Problem 76E
Related questions
Question
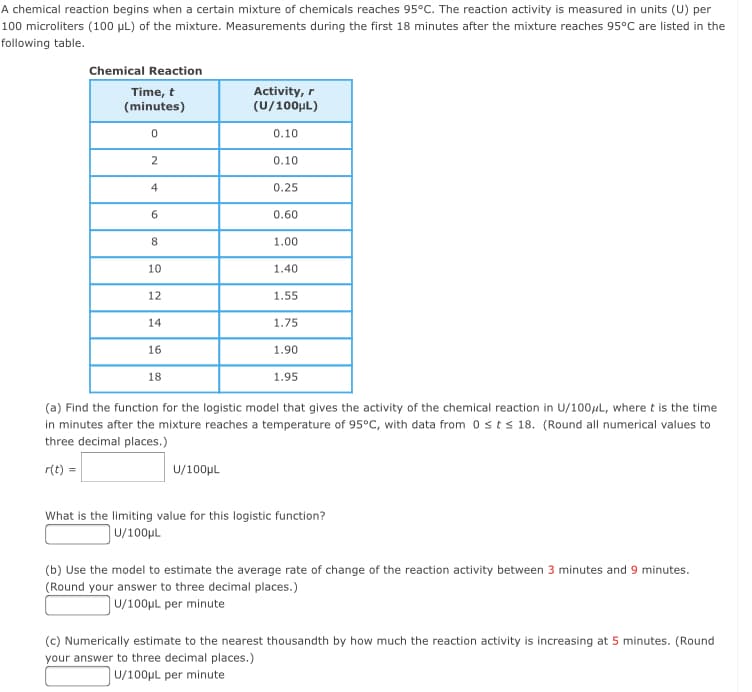
Transcribed Image Text:A chemical reaction begins when a certain mixture of chemicals reaches 95°C. The reaction activity is measured in units (U) per
100 microliters (100 µL) of the mixture. Measurements during the first 18 minutes after the mixture reaches 95°C are listed in the
following table.
Chemical Reaction
Time, t
(minutes)
Activity, r
(U/100μL)
0.10
0.10
0.25
6.
0.60
8.
1.00
10
1.40
12
1.55
14
1.75
16
1.90
18
1.95
(a) Find the function for the logistic model that gives the activity of the chemical reaction in U/100el, where t is the time
in minutes after the mixture reaches a temperature of 95°C, with data from 0sts 18. (Round all numerical values to
three decimal places.)
r(t) =
U/100µL
What is the limiting value for this logistic function?
U/100µL
(b) Use the model to estimate the average rate of change of the reaction activity between 3 minutes and 9 minutes.
(Round your answer to three decimal places.)
JU/100pL per minute
(c) Numerically estimate to the nearest thousandth by how much the reaction activity is increasing at 5 minutes. (Round
your answer to three decimal places.)
U/100µL per minute
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning

College Algebra (MindTap Course List)
Algebra
ISBN:
9781305652231
Author:
R. David Gustafson, Jeff Hughes
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning

College Algebra (MindTap Course List)
Algebra
ISBN:
9781305652231
Author:
R. David Gustafson, Jeff Hughes
Publisher:
Cengage Learning