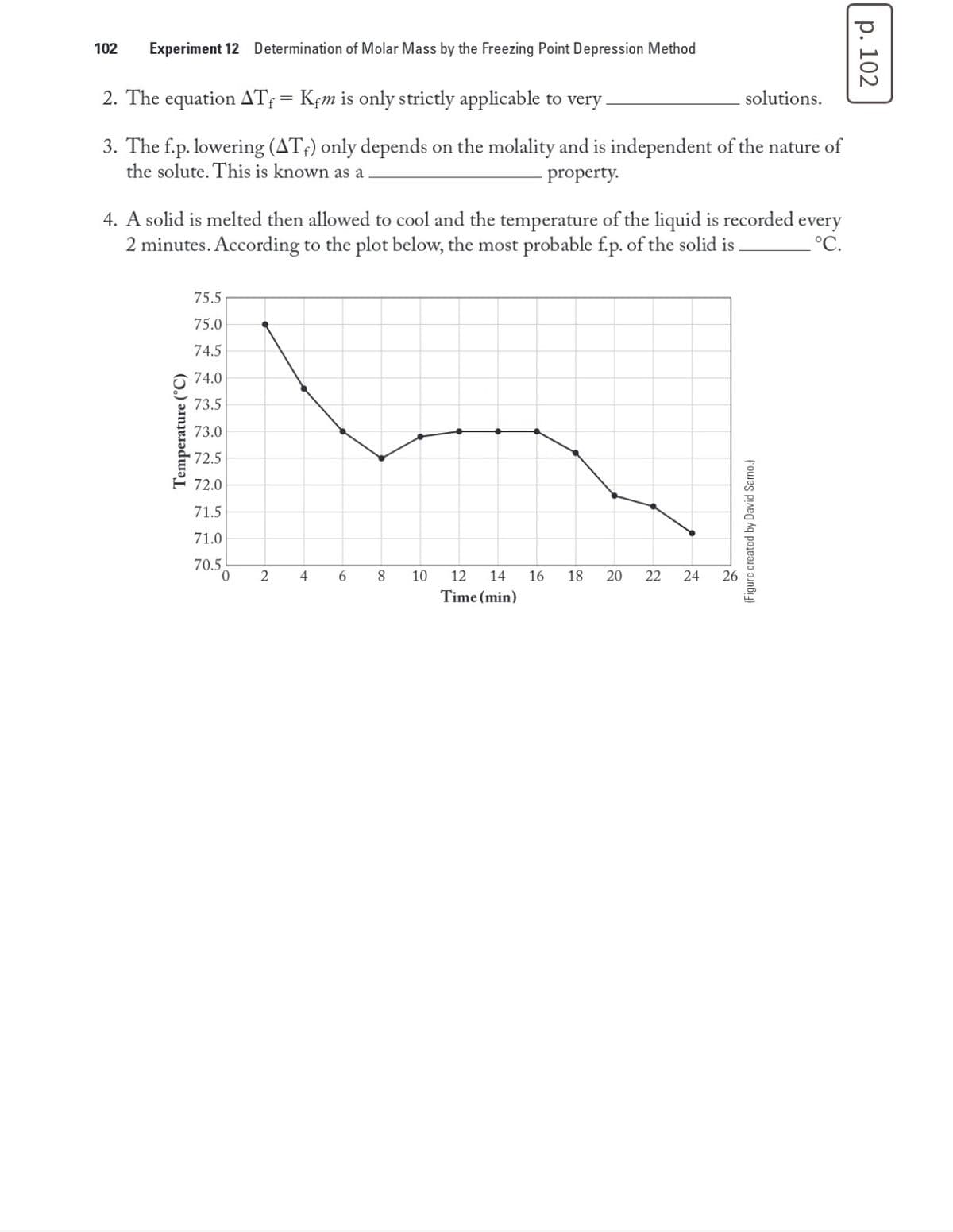
102 Experiment 12 Determination of Molar Mass by the Freezing Point Depression Method 2. The equation AT; = Kçm is only strictly applicable to very. solutions. 3. The f.p. lowering (AT;) only depends on the molality and is independent of the nature of the solute. This is known as a property. 4. A solid is melted then allowed to cool and the temperature of the liquid is recorded every 2 minutes. According to the plot below, the most probable f.p. of the solid is . °C. 75.5 75.0 74.5 74.0 73.5 73.0 72.5 72.0 71.5 71.0 70.5 4 6. 8. 10 12 14 16 18 22 24 26 Time (min) Temperature (°C) 20 (Figure created by David Samo.) р. 102
102 Experiment 12 Determination of Molar Mass by the Freezing Point Depression Method 2. The equation AT; = Kçm is only strictly applicable to very. solutions. 3. The f.p. lowering (AT;) only depends on the molality and is independent of the nature of the solute. This is known as a property. 4. A solid is melted then allowed to cool and the temperature of the liquid is recorded every 2 minutes. According to the plot below, the most probable f.p. of the solid is . °C. 75.5 75.0 74.5 74.0 73.5 73.0 72.5 72.0 71.5 71.0 70.5 4 6. 8. 10 12 14 16 18 22 24 26 Time (min) Temperature (°C) 20 (Figure created by David Samo.) р. 102
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 6RQ: In terms of Raoults law, distinguish between an ideal liquid-liquid solution and a nonideal...
Related questions
Question
Transcribed Image Text:102
Experiment 12 Determination of Molar Mass by the Freezing Point Depression Method
2. The equation ATf = Kfm is only strictly applicable to very
solutions.
3. The f.p. lowering (AT;) only depends on the molality and is independent of the nature of
the solute. This is known as a
property.
4. A solid is melted then allowed to cool and the temperature of the liquid is recorded every
2 minutes. According to the plot below, the most probable f.p. of the solid is
°C.
75.5
75.0
74.5
74.0
73.5
73.0
72.5
72.0
71.5
71.0
70.5
4
6.
10
12
14
16
18
20
22
24
26
Time (min)
Temperature (°C)
(Figure created by David Sarno.)
р. 102
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning
