107. Prepare a table like Table 4.2 for the four different isotopes of Sr that have the natural abundances and masses listed here. Sr-84 0.56% 83.9134 amu Sr-86 9.86% 85.9093 amu Sr-87 7.00% 86.9089 amu Sr-88 82.58% 87.9056 amu Use your table and the listed atomic masses to calculate the atomic mass of strontium.
107. Prepare a table like Table 4.2 for the four different isotopes of Sr that have the natural abundances and masses listed here. Sr-84 0.56% 83.9134 amu Sr-86 9.86% 85.9093 amu Sr-87 7.00% 86.9089 amu Sr-88 82.58% 87.9056 amu Use your table and the listed atomic masses to calculate the atomic mass of strontium.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 22E: An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99...
Related questions
Question
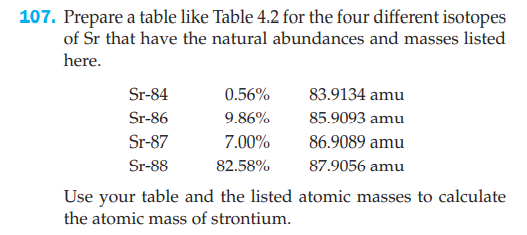
Transcribed Image Text:107. Prepare a table like Table 4.2 for the four different isotopes
of Sr that have the natural abundances and masses listed
here.
Sr-84
0.56%
83.9134 amu
Sr-86
9.86%
85.9093 amu
Sr-87
7.00%
86.9089 amu
Sr-88
82.58%
87.9056 amu
Use your table and the listed atomic masses to calculate
the atomic mass of strontium.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning