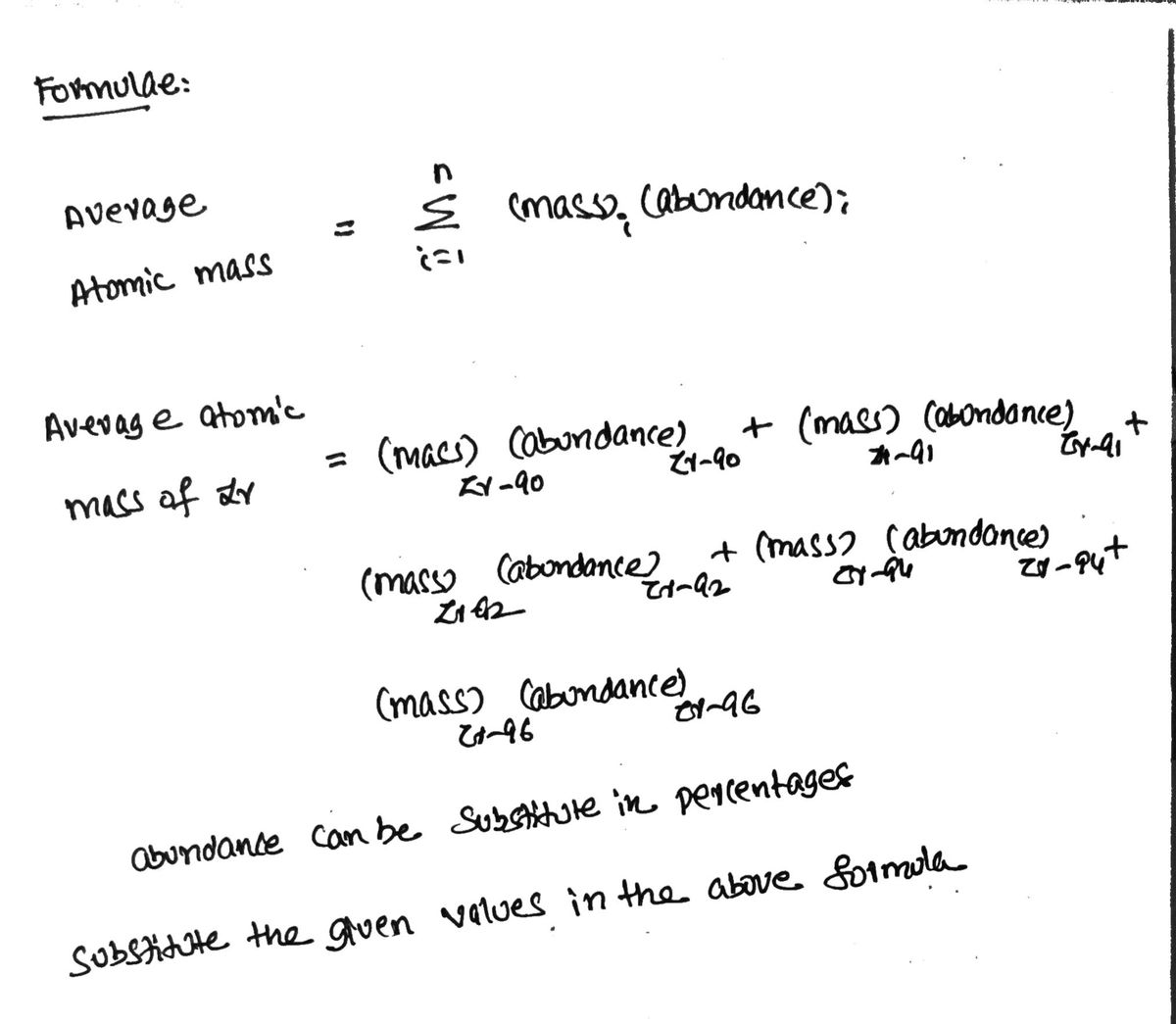
The element zirconium has five naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. Show your work to determine the average atomic mass. Isotope Mass % Abundance Zr-90 89.9043 51.40 Zr-91 90.9053 11.20 Zr-92 91.9046 17.10 Zr-94 93.9061 17.50 Zr-96 95.9082 2.80
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
The element zirconium has five naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. Show your work to determine the
Isotope Mass % Abundance
Zr-90 89.9043 51.40
Zr-91 90.9053 11.20
Zr-92 91.9046 17.10
Zr-94 93.9061 17.50
Zr-96 95.9082 2.80
Learn your way
Includes step-by-step video
Step by step
Solved in 2 steps with 2 images









