11%) Problem 9: The diagram presented represents a thermodynamic process experienced by 2.3 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal increments -782 cm³ while the pressure axis is divided into equal increments p = 0.251 atm. 4 po 3 po 2 po- I I I T I I I 1 I T 1 -T-1 I 1 יר־ו־ו־ז
11%) Problem 9: The diagram presented represents a thermodynamic process experienced by 2.3 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal increments -782 cm³ while the pressure axis is divided into equal increments p = 0.251 atm. 4 po 3 po 2 po- I I I T I I I 1 I T 1 -T-1 I 1 יר־ו־ו־ז
Related questions
Question
100%
Plz solve A b c , vll definitely upvote, handwritten or typed ... Thnku
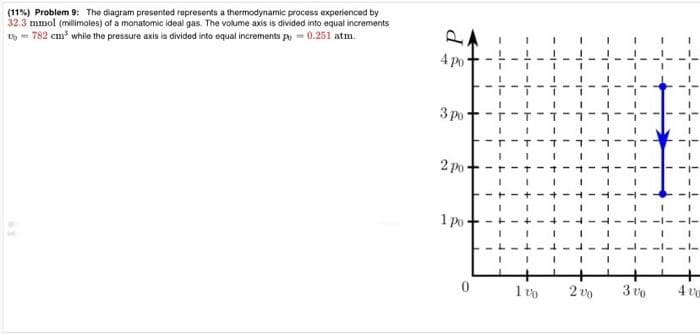
Transcribed Image Text:(11%) Problem 9: The diagram presented represents a thermodynamic process experienced by
32.3 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal increments
t=782 cm³ while the pressure axis is divided into equal increments p=0.251 atm
de
4 po
3 po
2 po
1 po
0
1
I
וו־ז־
1
-T-
1
120
-
I
I
1
1
I
-
I
7
200
-11
IL-L-
3.00
400

Transcribed Image Text:a) How much work, in joules, does the gas perform on its
environment during the thermodynamic process
represented in the diagram
B) What is the change, in joules, of the internal energy of
the gas during the process represented in the diagram?
C) How much heat, in joules, is absorbed by the gas
during the process represented in the diagram?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
