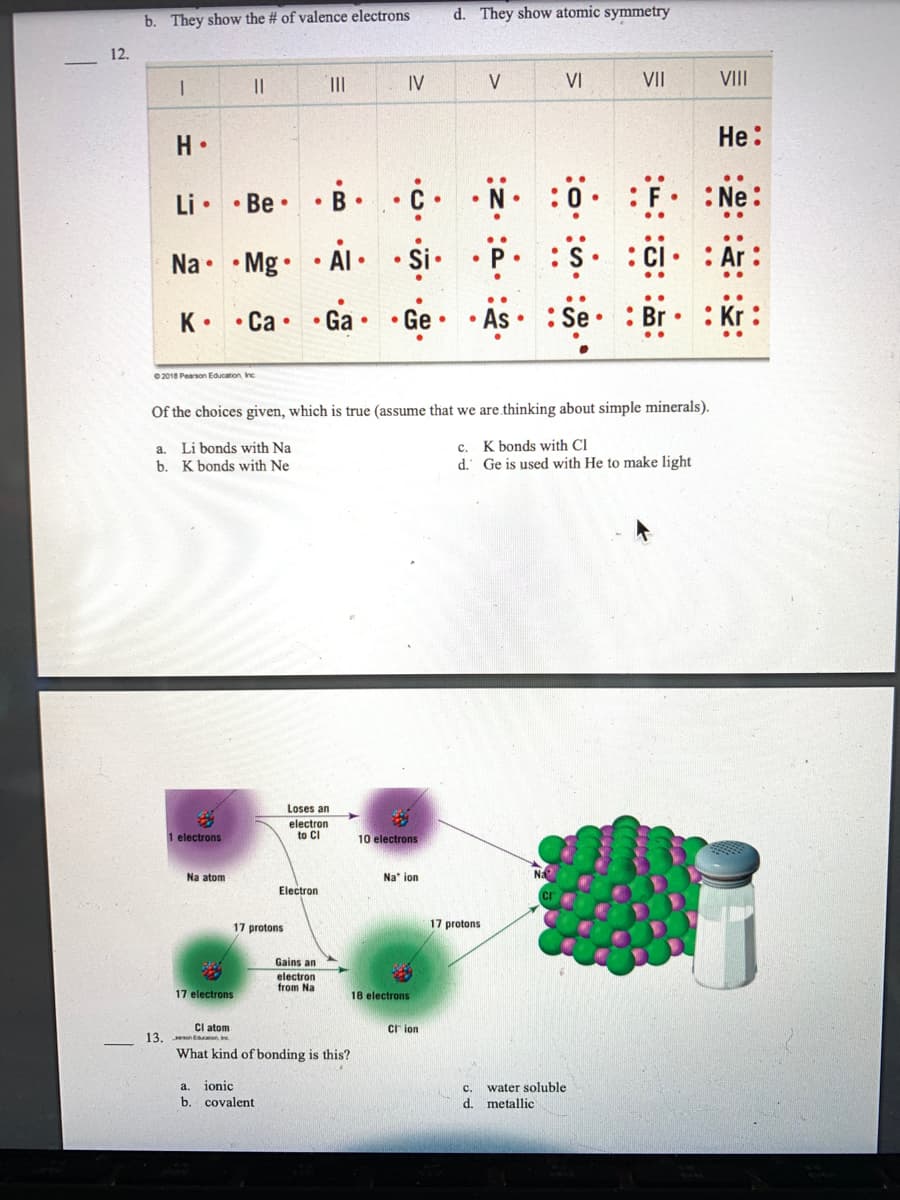
12. 13. • . 17 protons |||| ● I || H ● Li .Be. B. Na Mg. AI Si : Ar .. K .Ca. • Ga Ge . As Se : Kr ©2018 Pearson Education, Inc. Of the choices given, which is true (assume that we are thinking about simple minerals). a. Li bonds with Na c. K bonds with Cl b. K bonds with Ne d. Ge is used with He to make light 1 electrons Na atom Loses an electron to Cl Electron Gains an electron from Na 17 electrons Cl atom son Education be What kind of bonding is this? a. ionic b. covalent IV 10 electrons Na ion 18 electrons Cion ● V 17 protons VI :0 C. d. metallic water soluble VII : F. :CI. VIII He: .. Ne:
12. 13. • . 17 protons |||| ● I || H ● Li .Be. B. Na Mg. AI Si : Ar .. K .Ca. • Ga Ge . As Se : Kr ©2018 Pearson Education, Inc. Of the choices given, which is true (assume that we are thinking about simple minerals). a. Li bonds with Na c. K bonds with Cl b. K bonds with Ne d. Ge is used with He to make light 1 electrons Na atom Loses an electron to Cl Electron Gains an electron from Na 17 electrons Cl atom son Education be What kind of bonding is this? a. ionic b. covalent IV 10 electrons Na ion 18 electrons Cion ● V 17 protons VI :0 C. d. metallic water soluble VII : F. :CI. VIII He: .. Ne:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 87AP: At large interatomic separations, an alkali halide molecule MX has a lower energy as two neutral...
Related questions
Question
Please kindly assist me with # 12 and 13
Transcribed Image Text:-
12.
-
b. They show the # of valence electrons
1
||
|||
IV
VIII
H.
He:
..
Li..Be.
Ne:
..
..
..
..
Na Mg
• AI •
.Si. . p.
Ar
●
..
..
●
K .Ca. • Ga.
• Ge
●
As.
Se: Br
: Kr:
©2018 Pearson Education, Inc
Of the choices given, which is true (assume that we are thinking about simple minerals).
a. Li bonds with Na
c.
K bonds with Cl
b.
K bonds with Ne
d. Ge is used with He to make light
Na
1 electrons
Na atom
Electron
Gains an
electron
from Na
What kind of bonding is this?
a. ionic
b. covalent
17 electrons
Cl atom
13. son Education ne
Loses an
electron
to Cl
17 protons
10 electrons
Na ion
18 electrons
d. They show atomic symmetry
V
VI
VII
:0
:S :
Cion
17 protons
water soluble
C.
d. metallic
F.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning