12. Draw the Lewis Structures for each of the following molecules and ions by first clicking on the blue boxes and then on "edir. If you'd rather do this on paper, do so neatly and then paste a picture of your answer in place of the blue box. Either way works. h tewhs Stcu a. Ozone, O,
12. Draw the Lewis Structures for each of the following molecules and ions by first clicking on the blue boxes and then on "edir. If you'd rather do this on paper, do so neatly and then paste a picture of your answer in place of the blue box. Either way works. h tewhs Stcu a. Ozone, O,
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 64A
Related questions
Question
All of them is wrong can you please fix it for me, the instructions in the second picture
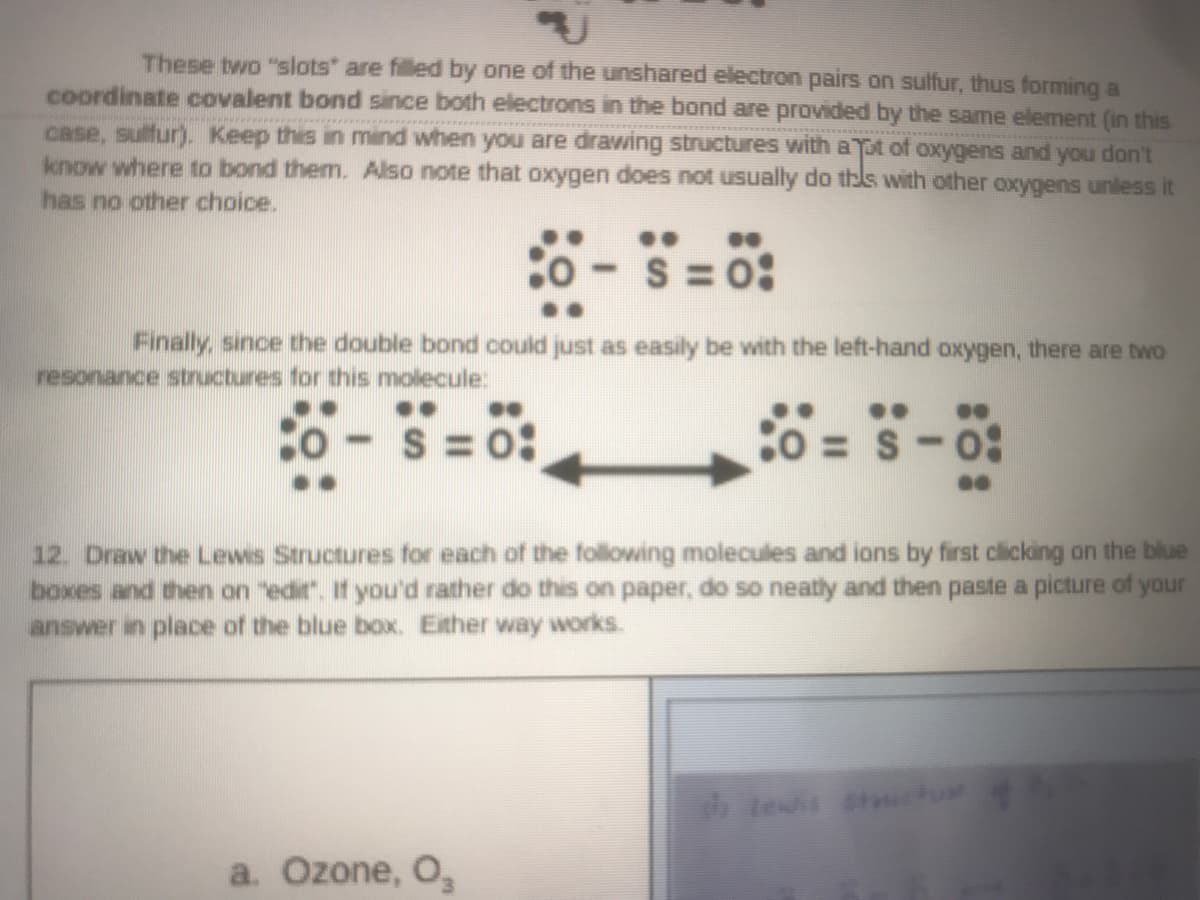
Transcribed Image Text:These two "slots" are filled by one of the unshared electron pairs on sulfur, thus forming a
coordinate covalent bond since both electrons in the bond are provided by the same element (in this
case, sulfur). Keep this in mind when you are drawing structures with aTpt of oxygens and you don't
know where to bond them. Also note that oxygen does not usually do thls with other oxygens unless it
has no other choice.
..
S = 0:
Finally, since the double bond could just as easily be with the left-hand oxygen, there are two
resonance structures for this molecule:
..
o -
S = 0:
= S-0:
12. Draw the Lewis Structures for each of the following molecules and ions by first clicking on the blue
boxes and then on "edir. If you'd rather do this on paper, do so neatly and then paste a picture of yaur
answer in place of the blue box. Exther way works.
a. Ozone, O,
Transcribed Image Text:Seturion
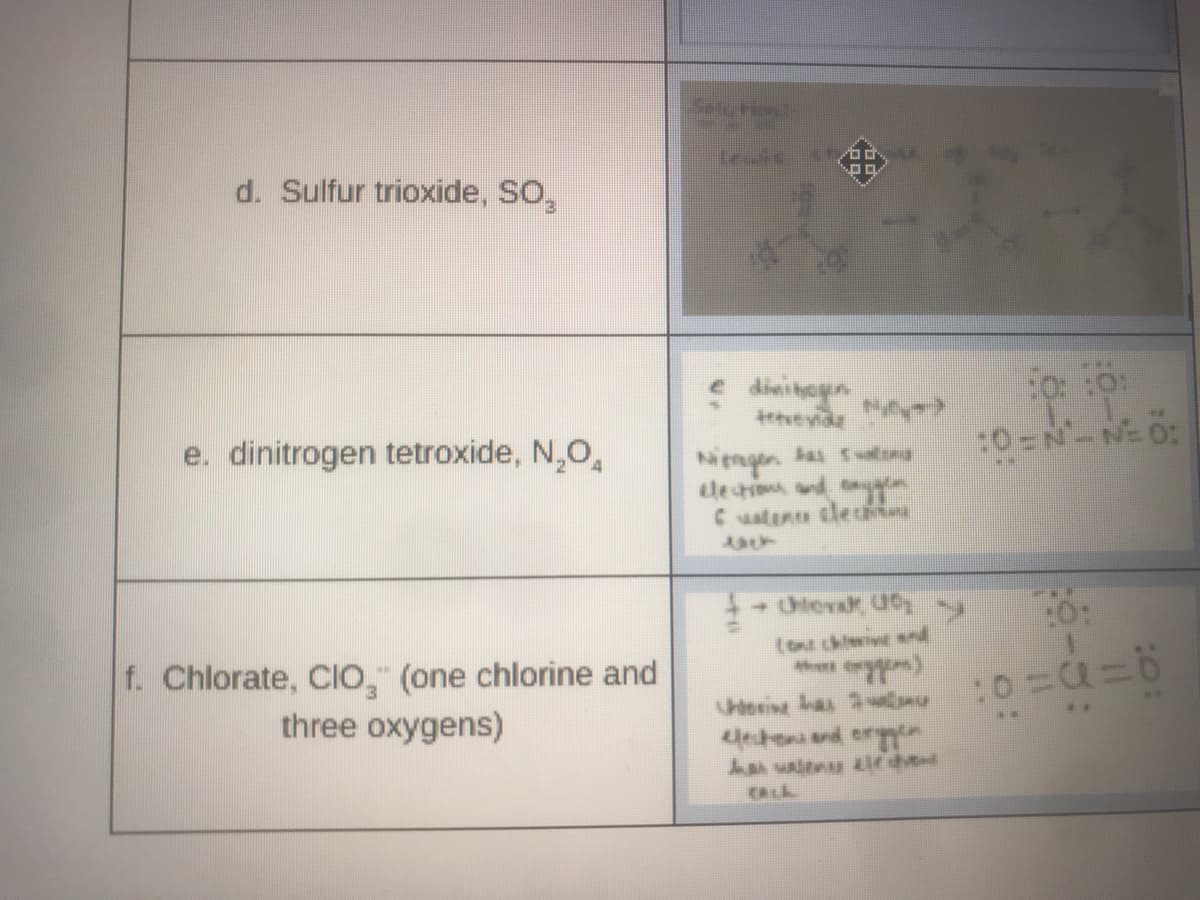
d. Sulfur trioxide, SO,
dini hayen
tetveyide
e. dinitrogen tetroxide, N,O,
:0=N-NE 0:
Nengen Sn
le tim and
Cuaten clec
to chiivt snd
f. Chlorate, ClO, (one chlorine and
three oxygens)
eris as 2AS
%3B
CALL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning