12. For the reaction Cl,0(g)+%O2(g) → 2C10, (g), AH° = 126.4 kJ/mol and AS° =-74.9 J/K mol. At 379°C, what is AG ? a. 154.8 kJ/mol b. 49.0 kJ/mol c. 175.2 kJ/mol d. 77.6 kJ/mol e. 157.3 kJ/mol
Q: For the reaction: %F(g) -- Fig), a reaction mixture initially contains 0.5 atm of F2(g) and 0.5 atm…
A: Given equilibrium is 1/2 F2(g) <------> F(g) Initial pressure of F2 , PF2 = 0.5 atm Initial…
Q: 6. Consider the reaction: CH,OH(/) + 30:(g) If the reaction mixture expands under constant pressure…
A:
Q: Consider the following reaction: CO2(g)+CCl4(g)⇌2COCl2(g) Calculate ΔG for this reaction at25 ∘C…
A: ∆Gr° = 2 × ∆Gf , COCl2 (g)° - ∆Gf , CO2 (g)° - ∆Gf , CCl4 (g)° = 2 x (-204.9) - (…
Q: Given the AH°f, calculate the AH°xn for the formation of diamond from graphite. AH°; (C, graphite) =…
A:
Q: Consider the reaction 2 NO2(g) N2O4(g) . (a) Using Gf N2O4(g) = 97.79 kJ/mol and Gf NO2(g) = 51.3…
A:
Q: Consider the reaction H2(g) + Cl2(g) 2HCI(g) AH = -384.6 kJ/mol. If 3 moles of H2 react with 3 moles…
A: Given data H2(g) + Cl2(g) → 2 HCl(g) ∆H=-384.6 KJ/mole Number of H2 mole react = 3 mole Number…
Q: At one time, a common means of forming small quantities ofoxygen gas in the laboratory was to heat…
A: Energy change takes place when reactants convert into product. The heat change of a reaction is…
Q: N22) + Oz) > 2NO) 2NO( + O26) → 2NO2() 2N20 → 2N2) + 02) use Hess's law to calculate AH for the…
A: The ∆H can be calculated as follows
Q: For the oxidation of solid elemental sulfur to gaseous sulfur dioxide S (s, rhombic) + O2 (g) -->SO2…
A:
Q: Calculate ∆Sº for the reaction below: N2(g)+2O2(g)⇄2NO2(g) where ∆Sº for N2(g), O2(g), & NO2(g),…
A: The given chemical equation is: N2(g)+2O2(g)⇌2NO2(g) The given standard entropy change (∆Sº) values…
Q: For 2-methylbutane, the ∆H° of vaporization is 25.22 kJ/mol and the ∆S° of vaporization is 84.48…
A: Given: The ∆H° of vaporization for 2-methylbutane = 25.22 kJ/mol The ∆S° of vaporization for…
Q: A 2.50 g block of iron is heated to 355.5 °C then plunged into 50.0 g of water at 25.0 °C. What will…
A: qw = -qm mw × cw × (Tf - Ti) = - { mm × cm ×( Tf-Ti) } 50 × 4.184 × (Tf - 25 ) = - { 2.50 × 0.444 ×…
Q: Consider the reaction, CuSO4(s) --><-- CuO(s) + SO3(g) if deltaGrxn = -14.6 kJ•mol^-1 at 950…
A:
Q: Calculate H for the reaction 2SO2(g) + 2P(s) + 5 Cl2(g) --> 2SOCl2(l) + 2POCl3(l) SOCl2(l) + H2O…
A:
Q: For the reaction N2(g) + 3 H2(g) = 2 NH3(g), what is AG (in kJ) at 298 K when the pressures of the…
A:
Q: 0.00258 mol of KI are dissolved into 40.0 g solution, the temperature of solution decreased by 1.57…
A: In a chemical reaction, some bonds are broken and some bonds are formed.this causes the heat change…
Q: Dissolution of Sodium Hydroxide mass of water: 200 g initial temperature of water: 20°C final…
A: Given: ΔH of NaOH dissolution: -44.53 kJ/mol To answer: The change in temperature when NaOH is…
Q: Consider the reaction:CH3OH( g)<--------->CO( g) + 2 H2( g)Calculate ΔG for this reaction at…
A:
Q: A 65.5 gram piece of glass (c 0.840 J/g C) is heated to 177"C and dropped into a beaker containing…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: The value of AG° at 100.0 °C for the formation of calcium chloride from its constituent elements: Ca…
A:
Q: A 65.5 gram piece of glass (c = 0.840 J/g C) is heated to 177°C and dropped into a beaker containing…
A: Since you have posted multiple questions, we will solve only the first question for you. To get…
Q: 2 A (g) + B (g) -> 2 C (s) ΔHº = ‒75.0 kJ ΔSº = ‒150.0 J/K ΔGº= -30.3 kJ iv. 0.100 atm of A,…
A: Given:- For this reaction :- 2 A (g) + B (g) ⇒ 2 C (s) ΔHº = ‒75.0 kJ ΔSº = ‒150.0 J/K ΔGº= -30.3…
Q: 18. A reaction has AH° = +38.7 kJ/mole, AS° = 112 J/mol K, and AG° = +5.32 kJ/mole. Complete the…
A: Given : ΔHo = 38.7 KJ/mol = 38700 ( Since 1 KJ = 1000…
Q: Consider the reaction 2 NO2(g) N2O4(g) . (a) Using Gf N2O4(g) = 97.79 kJ/mol and Gf NO2(g) = 51.3…
A:
Q: Using the provided table, determine the enthalpy for the reaction 2 K(s) + 2 H20(1) → 2 KOH (aq) +…
A:
Q: Hydrogen reacts with nitrogen to form ammonia (NH3) according to the reaction 3H2(g) + N2(g) -->…
A: Given: ∆Ho = -92.38 kJ/mol ∆So = -198.2 J/mol K Temperature (T) = 250°C
Q: Consider the generic reaction:A + 2 B -------> C + 3 D ∆H = 155 kJDetermine the value of ∆H…
A: Given generic equation is A + 2B → C + 3D Also ∆H for the above reaction=155kJ…
Q: Consider the following reaction: NiO (s) + CO (g) → Ni (s) +…
A: The partial pressure related with Gibbs free energy by the relations, ∆G=∆G0 +RTlnQp Where Qp…
Q: For the reaction CH4(g) + H2O(g)3H2(g) + CO(g) H° = 206 kJ and S° = 215 J/K G° for this reaction…
A:
Q: Consider the following reaction at 25 °C: 5 SO:(g) + 2 NH:(g) → 2 NO(g) + 5 SO:(g) + 3 H20(g) Given…
A:
Q: A metal pellet with mass 25.0 g and temperature 90.0 °C is dropped into 100.0 g water originally at…
A: Given: Mass of metal = 25.0 gm Initial temperature = 90.0 °C Mass of water = 100 gm Initial…
Q: Determine the AH° for the overall reaction based on the three-step thermochemical processes below:…
A: ∆H values - Reaction 1 = 167.5 KJ Reaction 2 = 341.4 KJ Reaction 3 = 43.4 KJ Final reaction = ?
Q: Calculate K, for H,0(g) + %0,(g)=H,0,(g) using the following data: H,(g)+ O,(g)=H,0,(g) K, = 2.3 ×…
A: H2g +O2 g ⇔H2O2 g..................1 Kp1 =2.3×1062 H2g +O2 g ⇔2H2O g................2 Kp2…
Q: For the following reaction: CS2(l) + 3 O2(g) →CO2(g) + 2 SO2(g) determine the constant pressure heat…
A: Given that - C(s) + O2(g) → CO2(g); ΔHf1=-393.5 kJ/mol .........(1) S(s) + O2(g) → SO2(g);…
Q: When determining the energy of a reaction where 0.250 mol of a substance with a AH = -2250.0 kJ/mol…
A: Energy of a reaction is either the energy released as a result of two reactant combining to give a…
Q: 11. Calculate AG° for the process, C(diamond) = C(graphite). Is the formation of graphite from…
A:
Q: For each of the following processes, indicate whether thesigns of ΔS and ΔH are expected to be…
A: The answer for part (a) is given below. Kindly repost the other subparts as separate question.
Q: Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and…
A: Given information: ∆H° = −56.13 kJ/mol ∆S° = 79.11 J/mol.K
Q: Which process would be predicted to have AS > 0? Periodic Table and Datasheet O Decomposition of a…
A: Entropy is defined as the degree of randomness. Greater the randomness greater will be the entropy.
Q: Consider the reaction 2NO 2( g) --> N 2O 4( g); Δ H° = –56.8 kJ and Δ S° = –175 J/K. In a container…
A: The question is based on the concept of chemical thermodynamics. we have to predict the position of…
Q: For each of the following processes would AS° be expected to be negative or positive? Why? 0:(g) +…
A: The Change in entropy is given by ∆S° which means how much is the disorder in the given reaction. If…
Q: What is AS° for the following reaction? 2C12(g) + SO2(g) – SOCI2{g) + Cl20(g) Substance: Cl2g) SO23)…
A:
Q: N2(g) + O2(g) 2 NO(g) H = +180.7 kJ 2 H2O(l) 2 H2(g) + O2(g) H = +571.7 kJ 4 NH3(g) + 5 O2(g)…
A: given, reaction 1: N2(g) + O2(g)⇔ 2 NO(g) H= +180.7 kJ reaction 2: 2 H2O(l) ⇔ 2 H2(g) + O2(g)…
Q: Consider the reaction: 2A(g) + B(g) → 2C(g) If ΔG° = −−5.4 kJ/mol at T = 25°C and PA = PB = 1 atm…
A: Given data Reaction : 2 A(g) + B(g) → 2 C(g) ∆G°=-5.4KJmol T=25°C PA=PB=1 atmPC=4 atm R=8.314…
Q: For the reaction: N2(g) + 2O2(g) + 16.2 kJ ➜ 2 NO2(g) As nitrogen reacts with oxygen, the heat of…
A: Nitrogen dioxide = N2O Heat of formation of N2 = 0 kJ Heat of formation of O2 = 0 kJ If heat is…
Q: Carbon monoxide, a toxic product from the incomplete combustion of fossil fuels, reacts with water…
A: Spontaneity of a reaction can be predicted by calculating the value of Gibbs free energy change. For…
Q: The value of ΔG° at 141.0°C for the formation of phosphorus trichloride from its constituent…
A:
Q: Consider the reaction: ICl(g) + Cl₂(g) → ICl₃(s). The ∆G° of the reaction is -17.09 kJ/mol.…
A:
Q: Hydrogen reacts with nitrogen to form ammonia (NH3) according to the reaction 3H2(g) + N2(g) --…
A: Given data: ∆H°= -92.38KJmol-1 ∆S°= -198.2Jmol-1K-1 Using equation: ∆G°= ∆H°- T∆S°
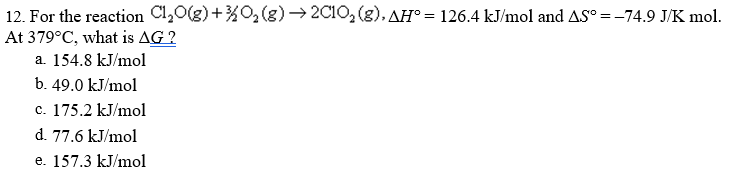
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 8 images

- What would be the equilibrium temperature (oC) if you drop 5.0 g of brass (Cp = 0.38 J/g-oC, initially at 68oC) and 3.5 g of aluminum (Cp = 0.9 J/g-oC, initially at 83oC) into 20 g of water (Cp = 4.184 J/g-oC, initially at 23oC)?The value of delta G at 281.0oC for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3CI2 (g) ---> 2PCI3 (g) is _____ kJ/mol. At 25.0oC for this reaction delta H is -720.5kJ/mol, delta G is -642.9 kJ/mol, and delta S is -263.7 J/K.10. What is ∆G for the decomposition of CaCO3 at 298K and a partial pressure of CO2 of 4*10-4 bar? ∆Gf0 for CaCO3(s), CaO(s) and CO2(g) are -1129 kJ/mol, -604 kJ/mol and -394 kJ/mol, respectively. CaCO3(s) → CaO(s) + CO2(g) A. 0 B. -131 kJ/mol C. 112 kJ/mol D. 131 kJ/mol E. 150 kJ/mol (Correct answer is C, I'm just looking for an explanation!).
- The value of Delta G at 261.0oC for the formation of phosphorous trichloride from its constituent elements, P2 (g) +3Cl2 (g) -> 2PCI3 (g) is ___ kJ/mol. at 25.0oC for this reaction, delta H is -720.kJ/mol, Delta G is -642.9kJ/mol and delta s is -263.7 J/K10.0 mol PCl3(g), 20.0 mol Cl2(g), and 40.0 mol PCl5(g) are introduced in a previously evacuated 10.0-L vessel at 25.0oC. The KP for the reaction at this temperature is 1.47×10–7: PCl5(g) →← PCl3(g) + Cl2(g), KP= 1.47×10–7 What is the immediate Gibbs free energy change for the reaction? In what direction the reaction will spontaneously approach equilibrium? Enter your answer with correct units and significant figures, and check the correct box below: Reaction proceeds spontaneously forward towards equilibrium. Reaction is at equilibrium. Reaction proceeds spontaneously backward towards equilibrium.A sample of graphite sealed under 0.458 atm of CO2(g) at 1080 K forms CO(g) and makes Ptot = 0.757 atm at eq≡m. Find KP: C(s) + CO2(g) 2 CO(g) KP = Peq(CO)2 Set up an ICE Table: Use other geven data to calculate the unknown: Ptot = 0.757 atm = Peq(CO2) + Peq(CO)= 0.458 – x + 2x = 0.458+x x=o.299 atm Peq(CO2) = 0.458 – x = ______ atm Peq(CO) = 2x = ______ atm Kp=Peq(CO)2 / Peq(CO2) = _____^2 atm2/ _____ atm = ______ atm
- a. Consider the data below for the reaction H2O(l) ⇌ H2O(g) . Plot a graph and determine ΔH and ΔS for the reaction. Describe how they influence the spontaneity of the reaction as a function of temperature. T (°C) P (torr) 0.0 4.579 10.0 9.209 20.0 17.53 25.0 23.76 30.0 31.82 40.0 55.32 60.0 149.4 70.0 233.7 90.0 525.8 Explain how the boiling point temperature of H2O(l) (at sea level) can be accurately determined from the data in a.? c. For the reaction in a., ΔE is less than ΔH. Explain.What is ∆G° for the reaction F₂(g) → 2 F(g) at 25.0 °C if K = 1.5 x 10⁻²²? (R = 8.314 J/mol・K)6. Which of the following reactions is a standard formation reaction at T = 298 K? a) C(s, graphite) + H2(g) + O2(g) → C6H12O6(s) b) 2 Fe (s) + 1.5 O2(g) → Fe2O3(s) c) N2(l) + 2 O2(l) → N2O4(l) d) Cl2(g) + H2(g) → 2 HCl(g) e) CO(g) + 2 H2(g) → CH3OH(l)
- The excessive production of ozone (O3) gas in the lower atmosphere causes rubber to deteriorate, green plants to turn brown, and persons with respiratory disease to have difficulty breathing. The reaction describing the formation of ozone from oxygen is shown below. 3 O2(g) <==> 2 O3(g) Calculate Grxn at 300 K for this reaction in a flask where [O2] = 1.179 x 10-2 M and [O3] = 7.973 x 10-3 M.Assume G° = 326 kJ. Use molarity to construct the reaction quotient, and express your answer in kJ.Determine whether the statements below are true or false. I: Kp and Kc are related through the ideal gas law. ["", ""] II: For equilibria where the number of moles of gaseous reactants and products are equal, Kc and Kp are numerically equal.Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 4.10 × 10⁻⁴ at a high temperature. If 0.20 mol N₂ and 0.15 mol O₂ react in a 1.0 L vessel, what will the equilibrium concentration of O₂ be? N₂(g) + O₂(g) ⇌ 2 NO(g) Set up the expression for Qc and then evaluate it to determine the direction of the reaction. Do not combine or simplify terms. Qc= (_______) / (_______) = (______) Based on the given values and your value for Qc, set up ICE table in order to determine the unknown. N₂(g) + O₂(g) ⇌ 2 NO(g) Initial (M) Change (M) Equilibrium (M) Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or simplify terms. Kc = (________) / (_______)= 4.10 × 10⁻⁴ Based on your ICE table and expression for Kc, solve for the…



