12. The combustion of glucose is represented by the following balanced equation: CgH1206 +6 O2 6 H20+6 CO2. Which reactant is the limiting reagent if there is 1 gram of both C6H2O6 and O2? a. CH1206 b. H20 с. Оз d. CO2
12. The combustion of glucose is represented by the following balanced equation: CgH1206 +6 O2 6 H20+6 CO2. Which reactant is the limiting reagent if there is 1 gram of both C6H2O6 and O2? a. CH1206 b. H20 с. Оз d. CO2
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
100%
Transcribed Image Text:I 596420-7 x
The impo X IG what ma X
H PAP Cher X
Qchapter
x
ぐ
->
b ar-2903012.agilixbuzz.com/student/135113422/activity/9ff34d34-df47-4fas022
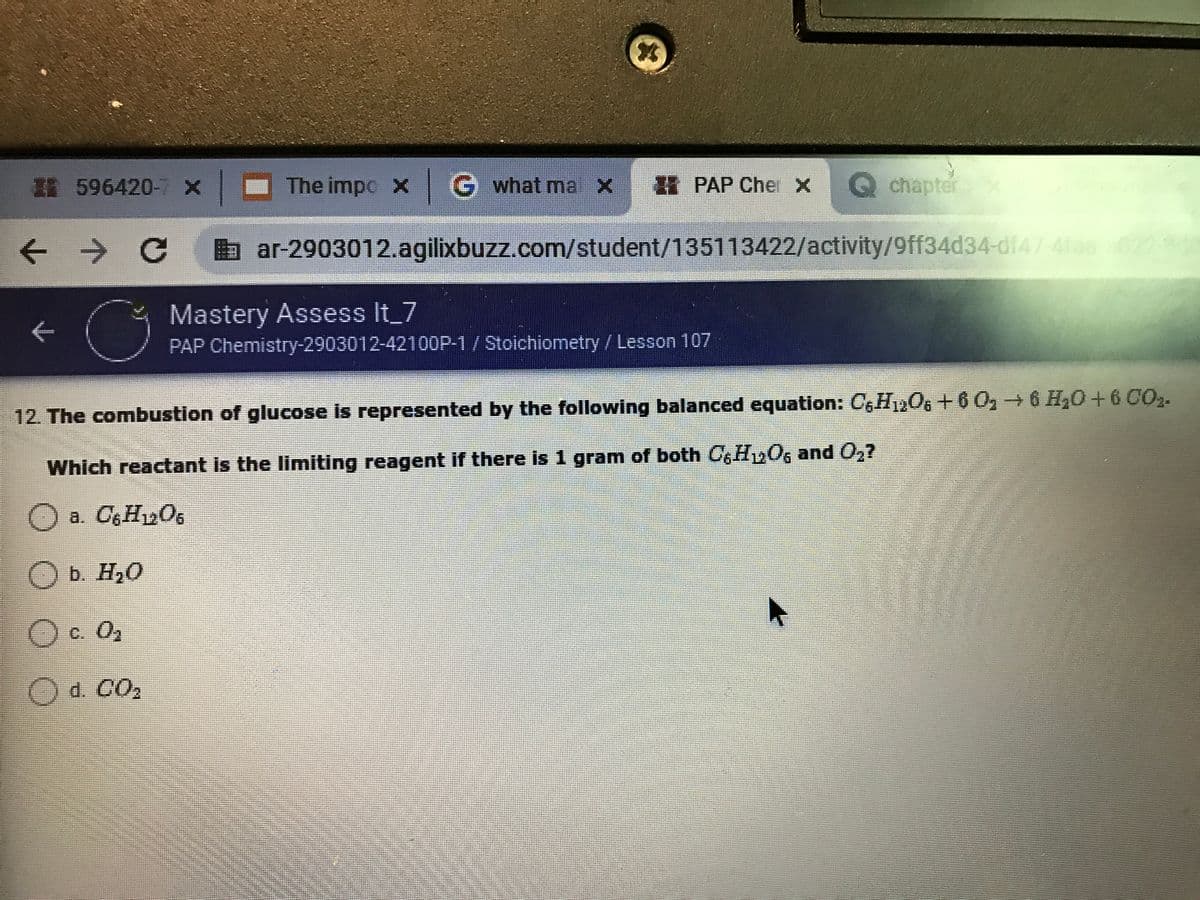
Mastery Assess It 7
PAP Chemistry-2903012-42100P-1/ Stoichiometry/Lesson 107
12. The combustion of glucose is represented by the following balanced equation: CGH12O6+6 0, 6 H20+6CO2.
Which reactant is the limiting reagent if there is 1 gram of both 0, Hy06 and 0,7
O a. CgHy06
O b. H20
O c. 0,
Od. CO,
Transcribed Image Text:596420 x
The imp X
G what mX
H PAP Ch x
1 Some r х
chapter x
G How m X
a ar-2903012.agilixbuzz.com/student/135113422/activity/9ff34d34-df47-4faa-a022-8d87ab138ea2
E PAP CI X
Chemis
Session
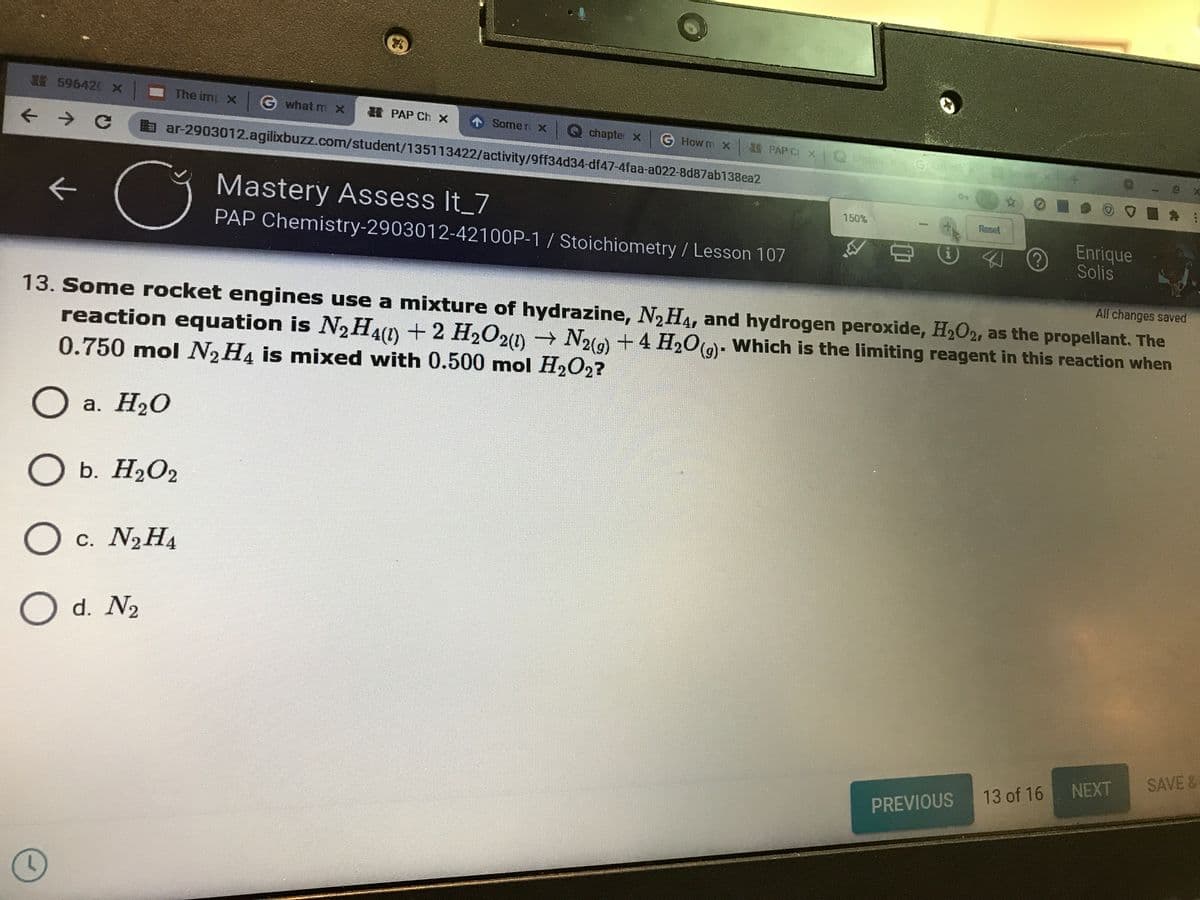
Mastery Assess It_7
150%
Reset
PAP Chemistry-2903012-42100P-1/ Stoichiometry / Lesson 107
Enrique
Solis
All changes saved
13. Some rocket engines use a mixture of hydrazine, N2H4, and hydrogen peroxide, H2O2, as the propellant. The
reaction equation is N2H4(1) +2 H2O20 N2)+4 H2O()- Which is the limiting reagent in this reaction when
0.750 mol N2H4 is mixed with 0.500 mol H2O2?
а. Н2О
O b. H2O2
O c. N2H4
d. N2
SAVE&
13 of 16 NEXT
PREVIOUS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

