123. The oxidation numbers of P, S, and Cl in H2PO2 , H2S, and KC104 are, respectively A) -1,-1, +3. B) +1,-2, +7. C) +1, +2, +7. D) -1, -2, +7. -1,-2, +3. E)
123. The oxidation numbers of P, S, and Cl in H2PO2 , H2S, and KC104 are, respectively A) -1,-1, +3. B) +1,-2, +7. C) +1, +2, +7. D) -1, -2, +7. -1,-2, +3. E)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter18: Oxidation–reduction Reactions And Electrochemistry
Section: Chapter Questions
Problem 7ALQ: In balancing oxidation-reduction equations, why is it permissible to add water to either side of the...
Related questions
Question
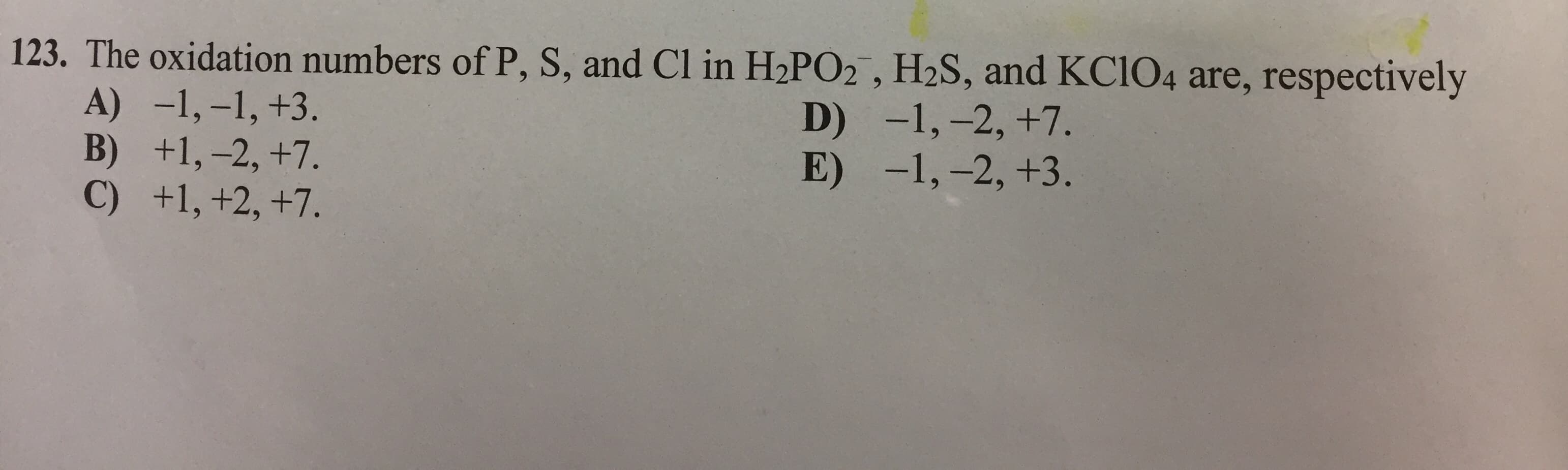
Transcribed Image Text:123. The oxidation numbers of P, S, and Cl in H2PO2 , H2S, and KC104 are, respectively
A) -1,-1, +3.
B) +1,-2, +7.
C) +1, +2, +7.
D)
-1, -2, +7.
-1,-2, +3.
E)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning