,1/3 The pressure change of a balloon depends on the equation P = C.V (C = 100kPa / m and n = -1 / 3). As the balloon is inflated with air at 25 °C (celciyus) temperature from 1m ^ 3 volume to 3m ^ 3 volume at 25 ° C (celciyus): Balloon Air 25 °C a)Find the mass (kg) of the air in the final state. P= C.V13 b)Find the work done by the air. (kJ) c)Show the work done on the P-v diagram.
,1/3 The pressure change of a balloon depends on the equation P = C.V (C = 100kPa / m and n = -1 / 3). As the balloon is inflated with air at 25 °C (celciyus) temperature from 1m ^ 3 volume to 3m ^ 3 volume at 25 ° C (celciyus): Balloon Air 25 °C a)Find the mass (kg) of the air in the final state. P= C.V13 b)Find the work done by the air. (kJ) c)Show the work done on the P-v diagram.
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter11: Fluid Statics
Section: Chapter Questions
Problem 68PE: During forced exhalation, such as when blowing up a balloon, the diaphragm and chest muscles create...
Related questions
Question
please solve the thermodynmicas question.
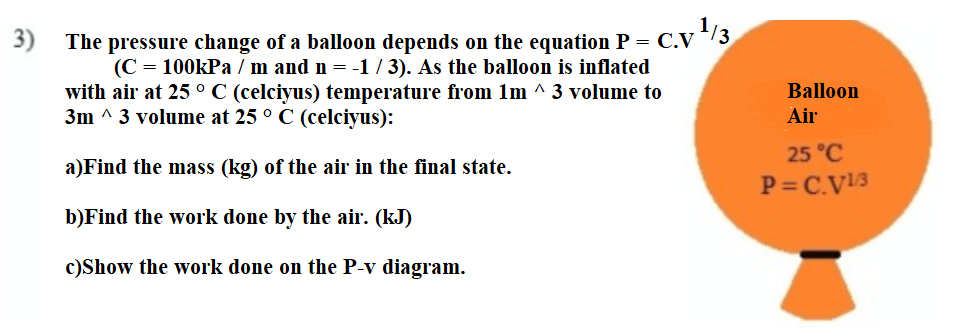
Transcribed Image Text:3)
The pressure change of a balloon depends on the equation P = C.V
(C = 100kPa / m and n = -1 / 3). As the balloon is inflated
with air at 25 °C (celciyus) temperature from 1m ^ 3 volume to
3m ^ 3 volume at 25 ° C (celciyus):
Balloon
Air
25 °C
a)Find the mass (kg) of the air in the final state.
P= C.V13
b)Find the work done by the air. (kJ)
c)Show the work done on the P-v diagram.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning