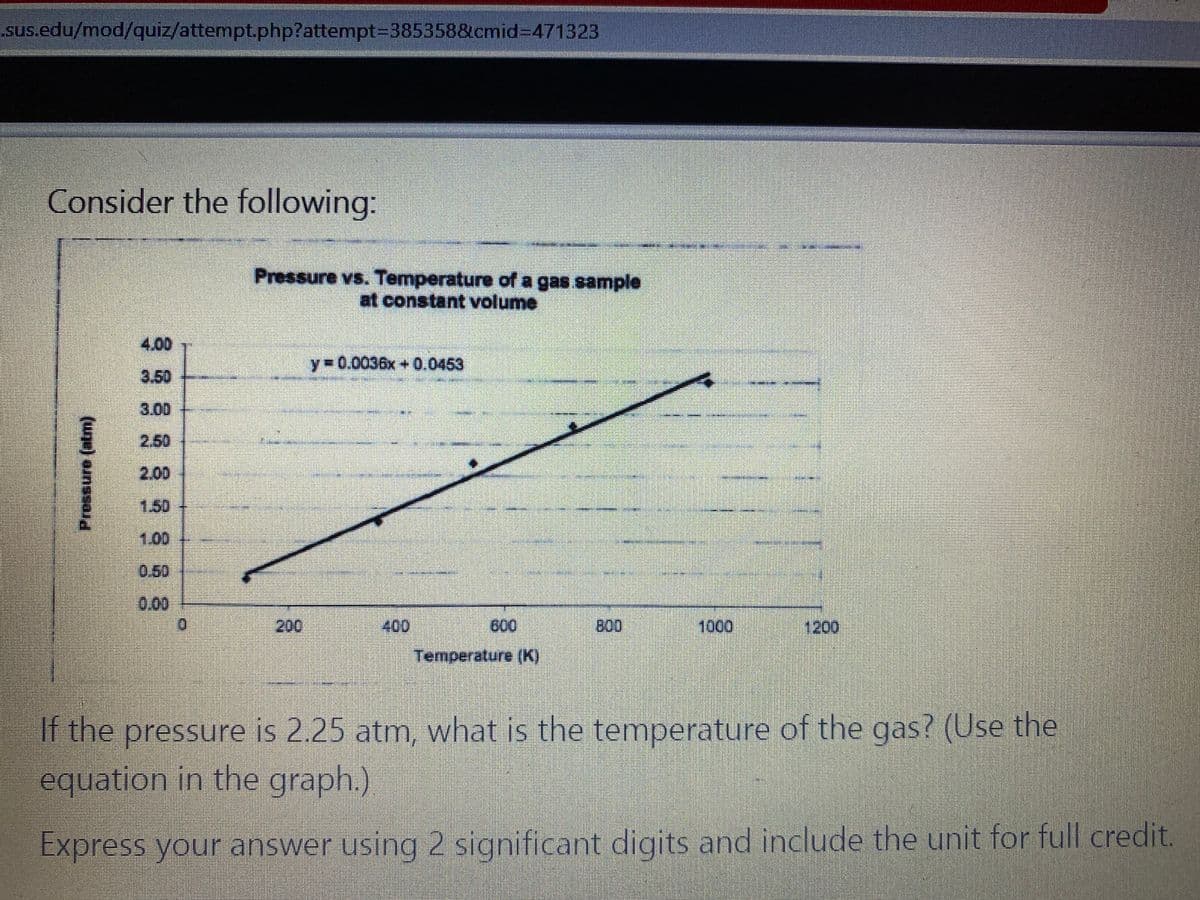
Consider the following: Pressure vs. Temperature of a gas sample at constant volume 4.00 y= 0.0036x + 0.0453 3.50 3.00 2.50 2.00 1.50 1.00 0.50 0.00 200 00 600 B00 1000 1200 Temperature (K) If the pressure is 2.25 atm, what is the temperature of the gas? (Use the equation in the graph.). Express your answer using 2 significant digits and include the unit for full credit. Pressure (atm)
Consider the following: Pressure vs. Temperature of a gas sample at constant volume 4.00 y= 0.0036x + 0.0453 3.50 3.00 2.50 2.00 1.50 1.00 0.50 0.00 200 00 600 B00 1000 1200 Temperature (K) If the pressure is 2.25 atm, what is the temperature of the gas? (Use the equation in the graph.). Express your answer using 2 significant digits and include the unit for full credit. Pressure (atm)
Astronomy
1st Edition
ISBN:9781938168284
Author:Andrew Fraknoi; David Morrison; Sidney C. Wolff
Publisher:Andrew Fraknoi; David Morrison; Sidney C. Wolff
Chapter5: Radiation And Spectra
Section: Chapter Questions
Problem 45E: If the emitted infrared radiation from Pluto, has a wavelength of maximum intensity at 75,000 nm,...
Related questions
Question
Transcribed Image Text:sus.edu/mod/quiz/attempt.php?attempt=385358&cmid%3D471323
Consider the following:
Pressure vs. Temperature of a gas sample
at constant volume
4,00
y-0.0036x +0.0453
3.50
3.00
2.50
2.00
1.50
1.00
0.50
0.00
200
400
600
800
1000
1200
Temperature (K)
If the pressure is 2.25 atm, what is the temperature of the gas? (Use the
equation in the graph.)
Express your answer using 2 significant digits and include the unit for full credit.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Astronomy
Physics
ISBN:
9781938168284
Author:
Andrew Fraknoi; David Morrison; Sidney C. Wolff
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Horizons: Exploring the Universe (MindTap Course …
Physics
ISBN:
9781305960961
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

Astronomy
Physics
ISBN:
9781938168284
Author:
Andrew Fraknoi; David Morrison; Sidney C. Wolff
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Horizons: Exploring the Universe (MindTap Course …
Physics
ISBN:
9781305960961
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning
