13. Now use the 10mL pipet to transfer 10 ml of maleic acid to each of the four 12 ME Erlenmeyer flask 14. Add 2 drops of methyl orange, and 2 drops of phenolphthalein to each flask. Rinse down the sides of the bottle with some DI water to get all acid into solution. (We don't nave to worry about changing the concentration since we know we transferred 10 mL of acid) 15. Repeat the titration procedure as before, using the initial run to learn the approximate volume you will need for the first and the second endpoint. Record initial volume readings, and stop at each endpoint to record volume readings from the buret. The first endpoint will be when the methyl orange in the flask turns a light orange color. Record the buret reading at the first endpoint. 16. Now continue adding NaOH to the flask. The solution should turn yellow as you add additional NaOH. The second endpoint will be when the phenolphthalein just begins to turn pink. The pink added to the yellow color of the methyl orange will create a light orange color in the flask. This is the second endpoint. Stop the titration and record the volume from the buret. If the solution turns a dark pink, you have gone past the endpoint. EXPERIMENTAL DATA: Include UNITS, UNCERTAINTY, and the correct use of SIGNIFICANT FIGURES 204.224glmdl Reaction 1, Standardization of NaOH: Test Run Run 1 Run 2 Run 3 Uncertainty 0. S399 0.000 95 ml. 0.00288md. 0.00ag4mdl. 0.603g 0.604 t0.0lg Exact Mass of KHP Moles of KHP Titration 23ML 283ML 20.2mL 0.0202L 0.0199L 2.0X10?L a.o x10~aL 9.3 mL to.0lml 303mL 29.3mlto.olml 19.9mL 20. omL 0.02L 2.0x10-2L Initial buret reading 10.4mL Final buret reading Volume (mL) NaOH delivered Volume (L) NAOH delivered 2.ox10-aL Example calculation using mol: mol conversion factor and ALL units mollL 0. 6o3mad 0.o१৭L 0.603md 0.02L 0.603 mol 21.9 M 30.3M 30.2m Molarity of NaOH: 30.1 Average Molarity NaOH: Bun l: Imol oH = 0.603ml OH - 603g KHP X * Just mally Avg? Yes! Blc 1:1 mol ratio. Reaction 2, Unknown maleic acid solution 4
13. Now use the 10mL pipet to transfer 10 ml of maleic acid to each of the four 12 ME Erlenmeyer flask 14. Add 2 drops of methyl orange, and 2 drops of phenolphthalein to each flask. Rinse down the sides of the bottle with some DI water to get all acid into solution. (We don't nave to worry about changing the concentration since we know we transferred 10 mL of acid) 15. Repeat the titration procedure as before, using the initial run to learn the approximate volume you will need for the first and the second endpoint. Record initial volume readings, and stop at each endpoint to record volume readings from the buret. The first endpoint will be when the methyl orange in the flask turns a light orange color. Record the buret reading at the first endpoint. 16. Now continue adding NaOH to the flask. The solution should turn yellow as you add additional NaOH. The second endpoint will be when the phenolphthalein just begins to turn pink. The pink added to the yellow color of the methyl orange will create a light orange color in the flask. This is the second endpoint. Stop the titration and record the volume from the buret. If the solution turns a dark pink, you have gone past the endpoint. EXPERIMENTAL DATA: Include UNITS, UNCERTAINTY, and the correct use of SIGNIFICANT FIGURES 204.224glmdl Reaction 1, Standardization of NaOH: Test Run Run 1 Run 2 Run 3 Uncertainty 0. S399 0.000 95 ml. 0.00288md. 0.00ag4mdl. 0.603g 0.604 t0.0lg Exact Mass of KHP Moles of KHP Titration 23ML 283ML 20.2mL 0.0202L 0.0199L 2.0X10?L a.o x10~aL 9.3 mL to.0lml 303mL 29.3mlto.olml 19.9mL 20. omL 0.02L 2.0x10-2L Initial buret reading 10.4mL Final buret reading Volume (mL) NaOH delivered Volume (L) NAOH delivered 2.ox10-aL Example calculation using mol: mol conversion factor and ALL units mollL 0. 6o3mad 0.o१৭L 0.603md 0.02L 0.603 mol 21.9 M 30.3M 30.2m Molarity of NaOH: 30.1 Average Molarity NaOH: Bun l: Imol oH = 0.603ml OH - 603g KHP X * Just mally Avg? Yes! Blc 1:1 mol ratio. Reaction 2, Unknown maleic acid solution 4
Chapter83: Simple Distillation
Section: Chapter Questions
Problem 2P
Related questions
Question
This is an exercise from a lab. The data is entered in the spaces and I am asked to find the molarity and average molarity of the base (NaOH)...is this correct? (See image below)....
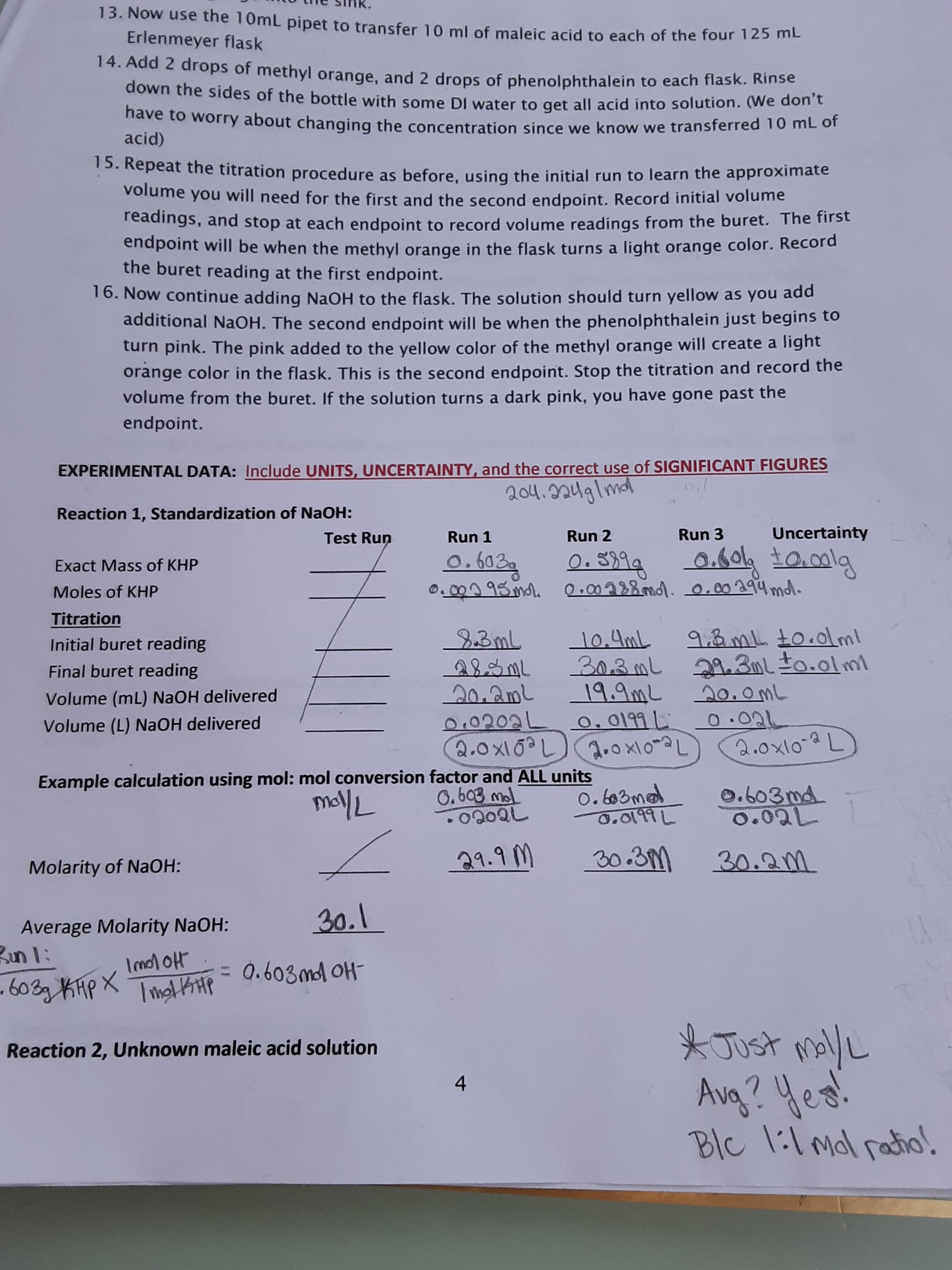
Transcribed Image Text:13. Now use the 10mL pipet to transfer 10 ml of maleic acid to each of the four 12 ME
Erlenmeyer flask
14. Add 2 drops of methyl orange, and 2 drops of phenolphthalein to each flask. Rinse
down the sides of the bottle with some DI water to get all acid into solution. (We don't
nave to worry about changing the concentration since we know we transferred 10 mL of
acid)
15. Repeat the titration procedure as before, using the initial run to learn the approximate
volume you will need for the first and the second endpoint. Record initial volume
readings, and stop at each endpoint to record volume readings from the buret. The first
endpoint will be when the methyl orange in the flask turns a light orange color. Record
the buret reading at the first endpoint.
16. Now continue adding NaOH to the flask. The solution should turn yellow as you add
additional NaOH. The second endpoint will be when the phenolphthalein just begins to
turn pink. The pink added to the yellow color of the methyl orange will create a light
orange color in the flask. This is the second endpoint. Stop the titration and record the
volume from the buret. If the solution turns a dark pink, you have gone past the
endpoint.
EXPERIMENTAL DATA: Include UNITS, UNCERTAINTY, and the correct use of SIGNIFICANT FIGURES
204.224glmdl
Reaction 1, Standardization of NaOH:
Test Run
Run 1
Run 2
Run 3
Uncertainty
0. S399
0.000 95 ml. 0.00288md. 0.00ag4mdl.
0.603g
0.604 t0.0lg
Exact Mass of KHP
Moles of KHP
Titration
23ML
283ML
20.2mL
0.0202L 0.0199L
2.0X10?L a.o x10~aL
9.3 mL to.0lml
303mL 29.3mlto.olml
19.9mL 20. omL
0.02L
2.0x10-2L
Initial buret reading
10.4mL
Final buret reading
Volume (mL) NaOH delivered
Volume (L) NAOH delivered
2.ox10-aL
Example calculation using mol: mol conversion factor and ALL units
mollL
0. 6o3mad
0.o१৭L
0.603md
0.02L
0.603 mol
21.9 M
30.3M
30.2m
Molarity of NaOH:
30.1
Average Molarity NaOH:
Bun l:
Imol oH
= 0.603ml OH
- 603g KHP X
* Just mally
Avg? Yes!
Blc 1:1 mol ratio.
Reaction 2, Unknown maleic acid solution
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT