14. A student hypothesizes that an increase in pressure will increase the amount of ammonia produced in the reversible reaction shown N2(g) + 3H2(9) 2NH3(g) - nitrogen hydrogen ammonia Which best explains how an increase in pressure increases the amount of ammonia present? O An increase in pressure favors the product with fewer particles. An increase in pressure tends to increase the temperature of the gases. O An increase in pressure would cause the forward reaction to be endothermic. O An increase in pressure decreases the rate of reaction between gases.
14. A student hypothesizes that an increase in pressure will increase the amount of ammonia produced in the reversible reaction shown N2(g) + 3H2(9) 2NH3(g) - nitrogen hydrogen ammonia Which best explains how an increase in pressure increases the amount of ammonia present? O An increase in pressure favors the product with fewer particles. An increase in pressure tends to increase the temperature of the gases. O An increase in pressure would cause the forward reaction to be endothermic. O An increase in pressure decreases the rate of reaction between gases.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.35QE
Related questions
Question
100%
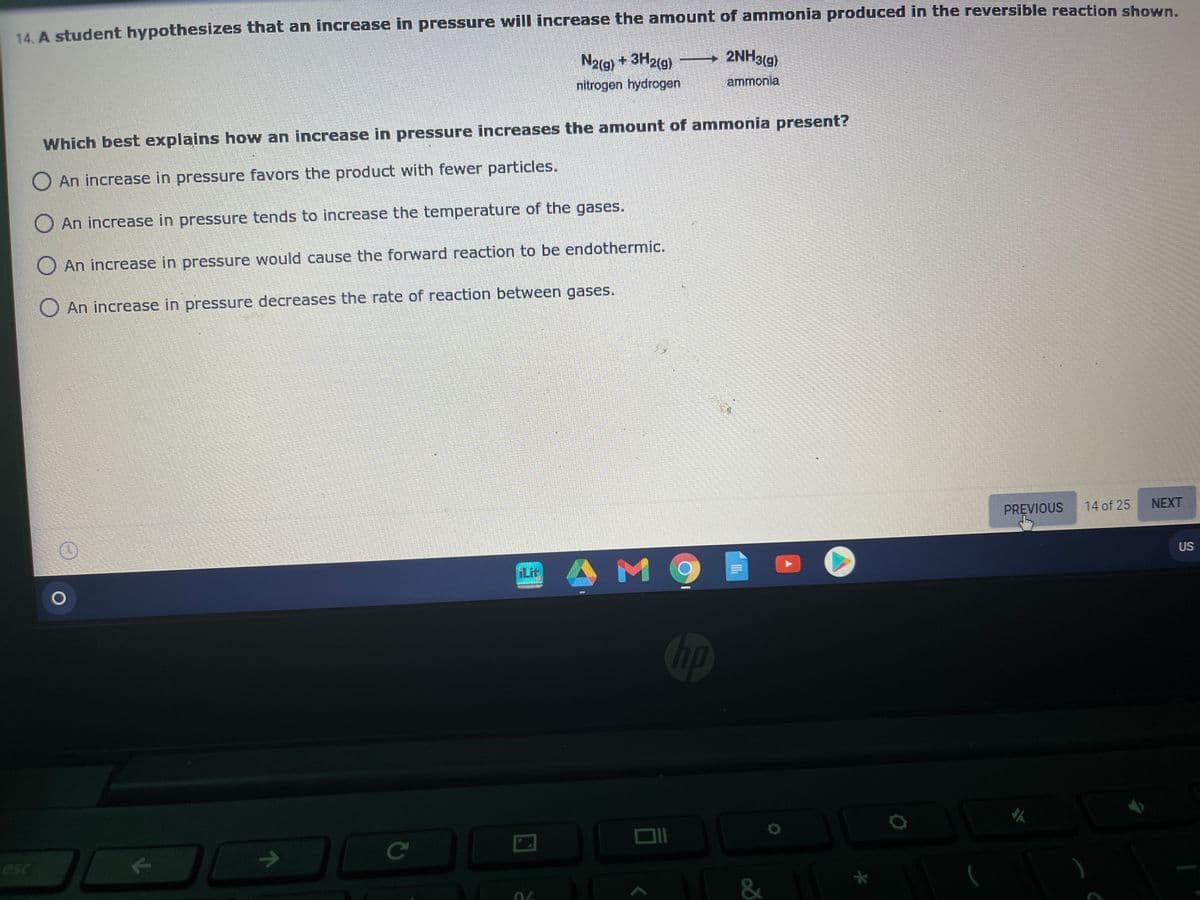
Transcribed Image Text:14. A student hypothesizes that an increase in pressure will increase the amount of ammonia produced in the reversible reaction shown.
2NH3(g)
N2(g) + 3H2(g)
nitrogen hydrogen
ammonia
Which best explains how an increase in pressure increases the amount of ammonia present?
O An increase in pressure favors the product with fewer particles.
O An increase in pressure tends to increase the temperature of the gases.
O An increase in pressure would cause the forward reaction to be endothermic.
An increase in pressure decreases the rate of reaction between gases.
PREVIOUS
14 of 25
NEXT
US
AM9
Lit
hp
ce
esc
&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning