The elementary reaction 2H2O(g)<-->2H2(g)+O2{g) proceeds at a certain temperature until the partial pressures of H20 , H2 , and O2 reach 0.053 atm, 0.0077 atm, and 0.0049 atm, respectively, at equilibrium. What is the value of the equilibrium constant at this temperature? Do not write your answer in scientific notation, give your answer as a decimal.
The elementary reaction 2H2O(g)<-->2H2(g)+O2{g) proceeds at a certain temperature until the partial pressures of H20 , H2 , and O2 reach 0.053 atm, 0.0077 atm, and 0.0049 atm, respectively, at equilibrium. What is the value of the equilibrium constant at this temperature? Do not write your answer in scientific notation, give your answer as a decimal.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.12E: 5.12. True or false: If all the partial pressures of reactants and products drop by half, the value...
Related questions
Question
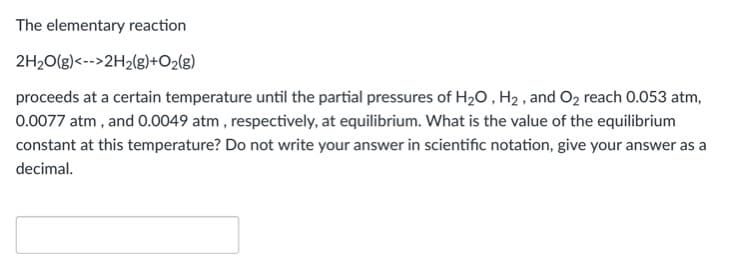
Transcribed Image Text:The elementary reaction
2H20(g)<-->2H2(g)+O2(g)
proceeds at a certain temperature until the partial pressures of H20 , H2 , and O2 reach 0.053 atm,
0.0077 atm , and 0.0049 atm , respectively, at equilibrium. What is the value of the equilibrium
constant at this temperature? Do not write your answer in scientific notation, give your answer as a
decimal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
