14.59 Denitrification occurs when nitrogen in the soil is lost to the atmosphere. One way in which denitrification can occur in acidic soils rich in plant matter is described by the following equation: C6H12O6(aq)+NO3(aq) CO2(g)+N2(g) Balance this equation, adding H*(aq) and H20(1) as necessary. ▼ Answer 24H*(aq)+5C6H12O6(aq)+24NO3(aq) 30CO2(g)+12N2(g)+42H2O(1)
14.59 Denitrification occurs when nitrogen in the soil is lost to the atmosphere. One way in which denitrification can occur in acidic soils rich in plant matter is described by the following equation: C6H12O6(aq)+NO3(aq) CO2(g)+N2(g) Balance this equation, adding H*(aq) and H20(1) as necessary. ▼ Answer 24H*(aq)+5C6H12O6(aq)+24NO3(aq) 30CO2(g)+12N2(g)+42H2O(1)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 37QAP: . Hydrogen gas and chlorine gas in the presence of light react explosively to form hydrogen chloride...
Related questions
Question
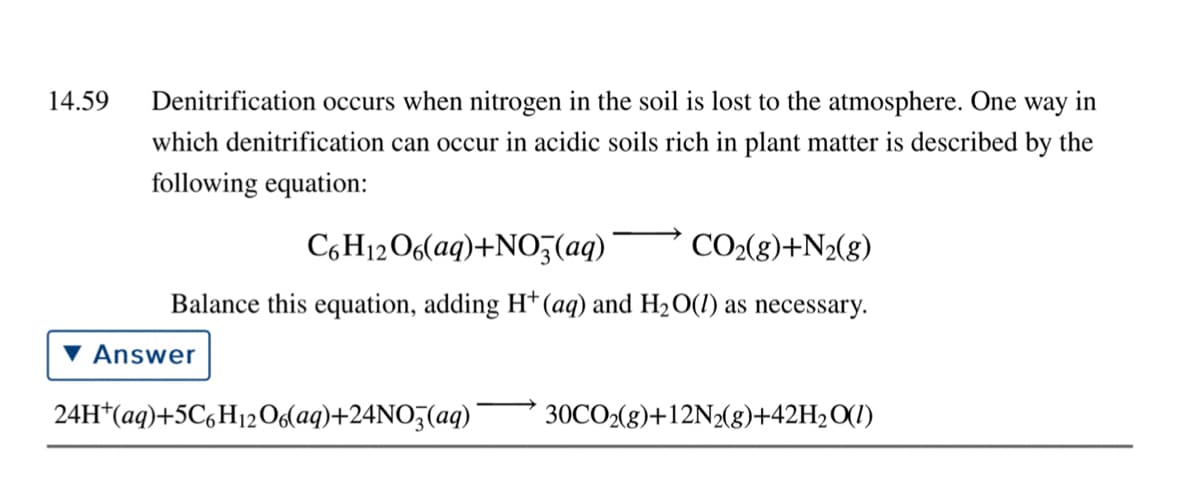
Transcribed Image Text:14.59
Denitrification occurs when nitrogen in the soil is lost to the atmosphere. One way in
which denitrification can occur in acidic soils rich in plant matter is described by the
following equation:
C6H12O6(aq)+NO3(aq)
CO2(g)+N2(g)
Balance this equation, adding H*(aq) and H2O(1) as necessary.
Answer
24H*(aq)+5C,H12O6laq)+24NO5(aq)'
30CO2(g)+12N2(g)+42H2O(1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning