15 18 21 P5 22 1.7 and greater 24 25 Cr Mn 15 23 28 30 NI Cu Extrem 31 32 Ga Ge 34 Se 35 Fe Co As 18 19 19 16 18 20 24 0.9 to 1.09 Strong 28 53 40 41 No 14 42 43 Mo To 45 44 Au 22 76 Os 22 47 Ag 22 48 49 in 17 51 50 Sn 52 Sb P3 0.4 to 0.89 Te 21 84 Po 19. Weak 12 18 18 22 19 17 16 19 2.5 74 72 Ts 25 Fe 77 29 Au 24 80 81 0.0 to .039 La 13 85 At Extrem TI 17 22 22 19 19 2.0 22 Eecnonigalana of the Elerierty Elements Difference In Strength of Bond Electronegativity Oxygen and Nitrogen Carbon and Hydrogen Ntragen and strantium Fluorine ard Sodium Carbon and Lthum Phosphorus and Hydrogen Chlorine and Potassium Prseric and Copper Carbon and Calcium Phosphorus and Calcum Bromne and Titanium Sulfur and Beryllium
15 18 21 P5 22 1.7 and greater 24 25 Cr Mn 15 23 28 30 NI Cu Extrem 31 32 Ga Ge 34 Se 35 Fe Co As 18 19 19 16 18 20 24 0.9 to 1.09 Strong 28 53 40 41 No 14 42 43 Mo To 45 44 Au 22 76 Os 22 47 Ag 22 48 49 in 17 51 50 Sn 52 Sb P3 0.4 to 0.89 Te 21 84 Po 19. Weak 12 18 18 22 19 17 16 19 2.5 74 72 Ts 25 Fe 77 29 Au 24 80 81 0.0 to .039 La 13 85 At Extrem TI 17 22 22 19 19 2.0 22 Eecnonigalana of the Elerierty Elements Difference In Strength of Bond Electronegativity Oxygen and Nitrogen Carbon and Hydrogen Ntragen and strantium Fluorine ard Sodium Carbon and Lthum Phosphorus and Hydrogen Chlorine and Potassium Prseric and Copper Carbon and Calcium Phosphorus and Calcum Bromne and Titanium Sulfur and Beryllium
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter22: Proteins
Section: Chapter Questions
Problem 22.65P: 22-65 (a) What is the difference in the quaternary structure between fetal hemoglobin and adult...
Related questions
Question
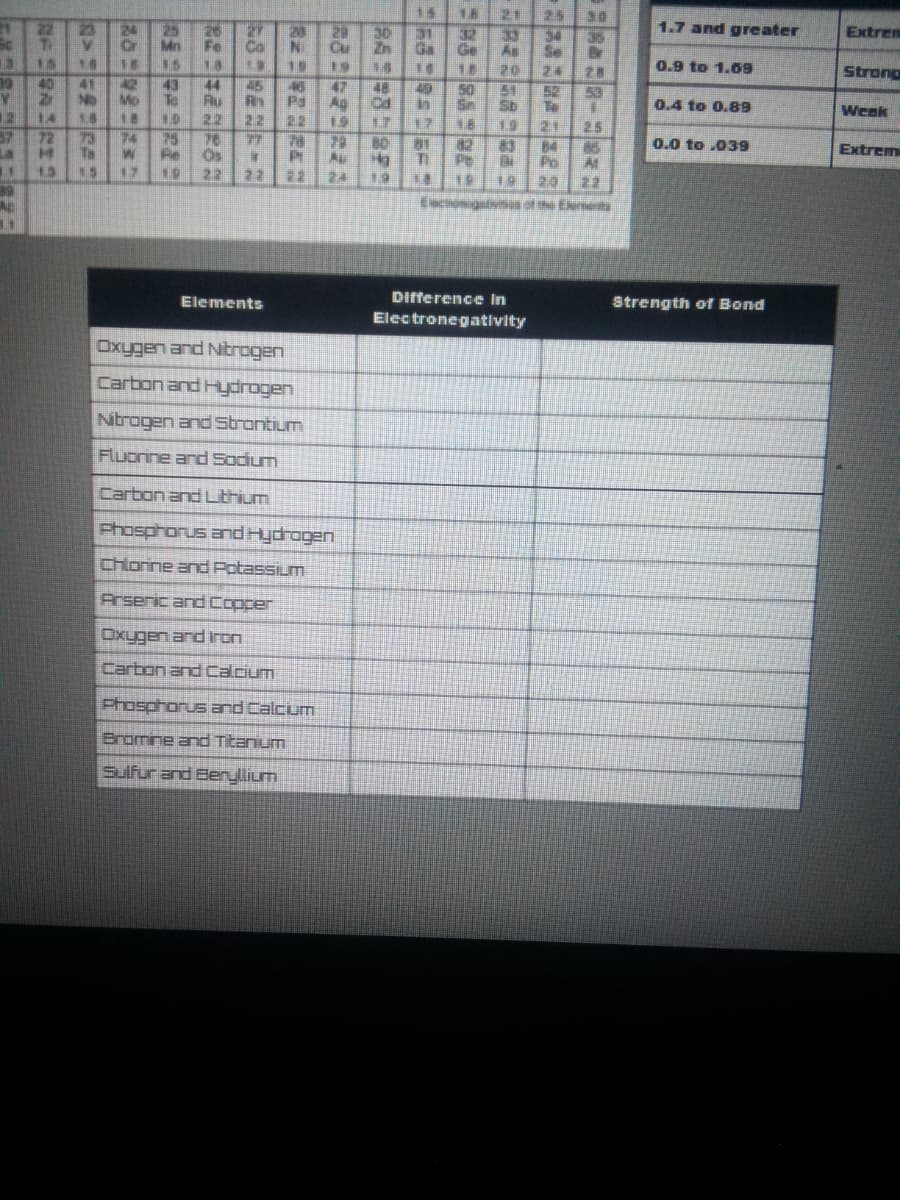
Transcribed Image Text:15
18
21
25
22
1.7 and greater
24
25
Cr
Mn
15
23
28
30
NI
Cu
19
32
Extren
34
Se
35
Fe
Co
Ga
Ge
As
16
18
18
19
16
18
20
24
28
0.9 to 1.09
Strong
GE
40
42
43
To
22
25
41
45
46
44
Au
48
47
Ag
19
49
50
51
52
53
No
Mo
Fd
in
Sn
Sb
Te
0.4 to 0.89
Wcak
12
72
La
14
1.8
18
1.9
22
22
77
17
17
16
19
21
2.5
74
76
Fe
29
Au
24
80
81
12
0.0 to .039
84
Po
19
Ta
Extrem
Os
22
TI
Pe
At
13
17
22
22
19
19
20
22
Eecnongalanaa of the Elernerts
Elements
Difference In
Strength of Bond
Electronegativity
Oxygen and Nitrogen
Carbon and Hydrogen
Ntragen and strantium
Fluorine and Sodium
Carbon and Lthium
Phosphorus and Hydrogen
Chlorine and Potassium
Prseric and Copper
Carbon and Calcium
Phosphorus and Calcum
Bromine and Titanium
Sulfur and Beryllium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning