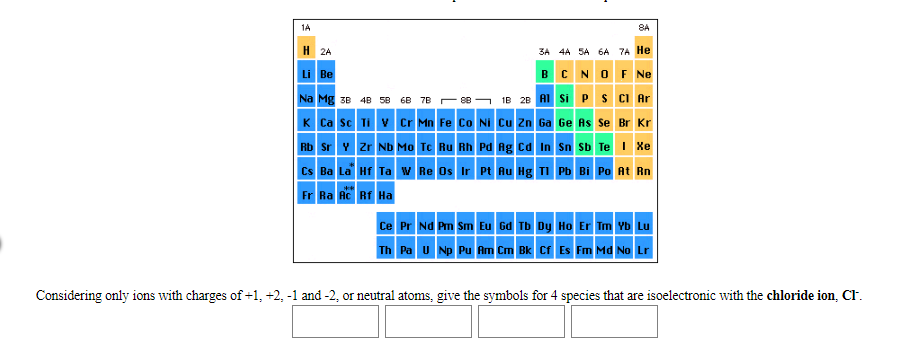
1A 8A H 2A 3A 4A SA 6A 7A He CNOF Ne Li Be В B 2B ASi PS CI Ar Na Mg 3B 4B 5B 6B 7B г 68 K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Xe Rb Sr Y Zr ND MO Tc Ru Rh Pd Ag ca In Sn Sb Te Cs Ba La Hf Ta Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Md No Lr Considering only ions with charges of+1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the chloride ion, Cl
1A 8A H 2A 3A 4A SA 6A 7A He CNOF Ne Li Be В B 2B ASi PS CI Ar Na Mg 3B 4B 5B 6B 7B г 68 K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Xe Rb Sr Y Zr ND MO Tc Ru Rh Pd Ag ca In Sn Sb Te Cs Ba La Hf Ta Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Md No Lr Considering only ions with charges of+1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the chloride ion, Cl
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.48PAE: A materials engineer has filed for a patent for a new alloy to be used in golf club heads. The...
Related questions
Question
Transcribed Image Text:1A
8A
H 2A
3A 4A SA 6A 7A He
CNOF Ne
Li Be
В
B 2B ASi PS CI Ar
Na Mg 3B 4B 5B
6B 7B г 68
K Ca Sc TiV
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Xe
Rb Sr Y Zr ND MO Tc Ru Rh Pd Ag ca In Sn Sb Te
Cs Ba La Hf Ta Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa UNp Pu Am Cm Bk Cf Es
Md No Lr
Considering only ions with charges of+1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the chloride ion, Cl
Expert Solution
Step 1
Isoelectronic species contains same number of electrons in each species.
The atomic number of chlorine is 17. It contains 17 electrons in monoatomic species.
The number of electrons present in chloride ion, Cl- is 18.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning