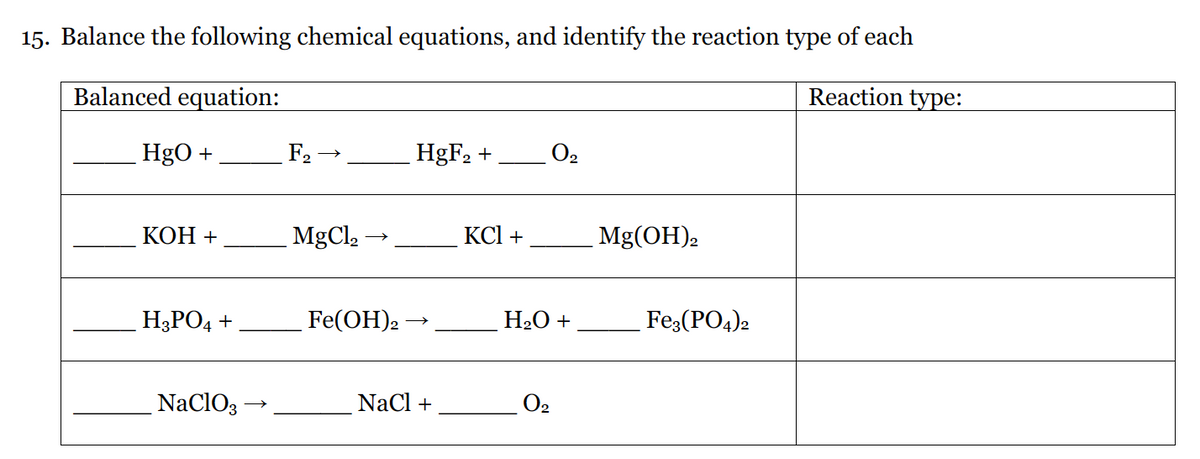
15. Balance the following chemical equations, and identify the reaction type of each Balanced equation: Reaction type: HgO + F2 → HgF2 +, O2 КОН + MgCl2 KCl + Mg(OH)2 H3PO4 + Fe(ОН). H2O + Fe:(PO4)2 NaClO3 NaCl + O2
Q: 4. H2SO4 + Al(OH)3 -----> Al2(SO4)3. + H2O
A: When sulfuric acid reacts with aluminium hydroxide products are aluminium sulfate and water. H2SO4…
Q: Balance the following chemical equation and determine the mass in grams of FeO(s) needed to produce…
A: The reaction given is, => FeO (s) + C (s) → Fe (s) + CO2 (g) Given: Mass of Fe to be produced =…
Q: A 0.5962 g sample of iron ore is dissolved in perchloric acid (HClO4 ). All iron present is oxidized…
A:
Q: Write “true” or “false” for each statement. (a) We balancechemical equations as we do because energy…
A: The given statement is false. The chemical equations are balanced for satisfying the law of…
Q: 12. Explain the stoichiometry (mole ratio of reactants) of this reaction. OH 3 + BH3 b. H2O2, OH
A: The given reaction is the hydroboration of but-2-ene. It is an oxidation reaction.
Q: Lithium metal reacts with nitrogen gas to form lithium nitride. Identify the balanced reaction that…
A:
Q: Balance the following reaction in acidic conditions: I2 + HNO3 → 2 HIO3 + 10 NO2 What are the…
A: The reaction given is I2 + HNO3 → HIO3 + NO2 In the above reaction, initially I in I2 is in 0…
Q: How do you balance this chemical equation? Fe(OH)2 + O2 + H2O ---> Fe(OH)3
A:
Q: KCI H2 + F2 HF 9. Fe + O2→ Fe:O4 10. Mg + HCI → MgCl2 8. +. + H2
A: 4 reactions are given . We have to balance them and identify the type of reaction . When two species…
Q: The reaction between elemental phosphorus P4(s) and O2(g) to make P4010(s):
A: A balanced equation is an equation in which number of atoms for each element in reactants same to…
Q: A. The reaction between ammonia and oxygen is given below: 2 NH3(g) + 2 02(g) → N,0(g) + 3 H20(1) We…
A: Given reactions are : Which of the following reactions can also occurs = ?
Q: What is the sum of the balancing coefficients when properly balanced? Write a balanced equation for…
A:
Q: phosphate à Iron (II) phosphate + K2SO4 Balance the reaction.?
A: Balanced chemical reaction number of atoms of each element in product side is equal to number of…
Q: 4. Balance the following reactions, using coefficients, and label the type of reaction (combination,…
A:
Q: NaF + Br2 -> NaBr + F2 What type of reaction is this (choices are Synthesis, Decomposition, Single…
A: In balanced chemical reaction number of each atom on reactant side is equal to number of each atom…
Q: alculate the theoretical yield of (in grams) of Ca3(PO3)2 when 7.15 g of calcium hydroxide reacts…
A: The balanced equation is: 3Ca(OH)2 + 2H3PO4 → 6H2O + Ca3(PO3)2 The given mass of Ca(OH)2 = 7.15 g…
Q: ( P 4 ) is commercially prepared by heating amixture of calcium phosphate (CaSi O 3 ), sand (SiO 2…
A: Given that : Mass of Ca3(PO4)2 = 250.0g Mass of SiO2 = 400.0 g Yield of P4 =? Percent yield of P4 =?…
Q: What mass of silver nitrate, AgNO3, is requiredto prepar 800 g f350% solution of AgNO3 ? a. 24.6 g…
A: The required mass of Silver nitrate can be calculated as3.50% AgNO3 means 3.50g AgNO3/100 g soln.
Q: What is the sum of ALL coefficients when the following equation is balanced, using the smallest…
A: The balanced equation can be written as: 4Ag + 2H2S + 1O2 → 2Ag2S + 2H2O When 4 mole Ag reacts with…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Given, Cr2(SO4)3 (aq) + (NH4)2CO3 (aq) Required,…
Q: S8(k) + O2(g) --> SO3(g) What can be the coefficient of oxygen gas
A:
Q: S₈(s)+Na₂So₃(aq)+H₂O(℩)→Na₂S₂O₃·5H₂)(s) (unbalanced) What is the theoretical yield of…
A: The theoretical yield of a substance is the expected yield that is found by calculation using the…
Q: 6. The element carbon undergoes many inorganic reac- tions, as well as being the basis for the field…
A: Since you have posted multiple question with multiple sub parts, we will solve first three sub-parts…
Q: balance this chemical reaction ____ CaCO3 + ____ HCl → _____- CaCl2 +…
A: According to the Law of conservation of mass " all atoms of different elements must be equal on both…
Q: Q2. a) What is Atom Economy? Calculate Atom Economy for the following reactions, i)…
A: Given, Atom economy Synthesis of 1-bromobutane Preparation of hydrogen Required,…
Q: 5. K2SO4 + BaCL2 --> KCL + BaSO4 -----
A: K2SO4+ BaCl2 -------> KCl + BaSO4
Q: Identify the type of equation represented by the reaction below. C3H5(NO3)3 → CO2 + N2 + H20 + O2 O…
A: Explanation to the correct answer is given below
Q: Predict the products of the following reaction, including correct physical states, and balance the…
A: Concept: This is a ionic double displacement reaction. In a double-replacement reaction two ionic…
Q: The reaction between ammonia and oxygen is given below: 2 NH3(g) + 2 O2(g) N2O(g) + 3 H2O(l) We…
A: If the reaction is reversible, the reverse reaction can also occur.
Q: 5. (a) What do you mean by 'atom economy' of a reaction? How does atom economy differ from % yield?…
A:
Q: How many metric tons of charcoal is required to reduce 1500 kg of arsenic trioxide? AS2O3 + C = CO +…
A: Since you have posted multiple questions, we are entitled to answer the first only. 1) The reaction…
Q: Complete and balance the following chemical equations: H2O (l) + P2P5 --> Li2O (s) + H2O (l) -->
A:
Q: Complete and balance the following chemical equation: Ni (s) + H2SO4 (ap) -->
A: In electrochemical series, Ni is present above H. Hence, Ni can acts as reducing agent means Ni will…
Q: Balance the following reactions: A. Pb(CH3CO0)2 + Na2SiO3 → PbSiO3 + NaCH3COO B. C2H5OH + 02 CO2 +…
A: According to the Law of conservation of mass " all atoms of different elements must be equal on both…
Q: 2 Balance the following equations and indicate whether they are combination, decomposition, or…
A: Note : As per our company guidelines we are supposed to answer only first 3 sub-parts. Kindly repost…
Q: Determine the physical states for the products and then determine which factor does NOT drive…
A: (i): Since KOH is a soluble salt, it is completely soluble in water. Hence state of KOH is aqueous…
Q: Give the balanced equation for each of the following chemical reactions:a.Glucose (C6H12O6) reacts…
A: Change in the matter can be divided into two categories, one is physical change and other is…
Q: Which element in the compounds below has an oxidation number (state) of +2? N in NH4Br Ba in…
A: We have to predict the compound that have element in +2 oxidation number.
Q: Balance the chemical reaction below: _Pb(OH)2 + _NaCl => _PbCl2 + _NaOH…
A:
Q: 5. (a) What do you mean by 'atom economy' of a reaction? How does it differ from E-factor? Calculate…
A:
Q: Which of the following best describes why MnSO4→ MnO + SO3 is not a synthesis reaction? Oxygen is…
A: Synthesis reaction is a type of reaction in which two or more reactants combine to form a single…
Q: 6- In the reaction below by complete reaction of 2 mol KCIO3, 120.6 g KCl is produced. Calculate the…
A: In the given reaction KClO3 decomposes into KCl and O2. The equation for the balanced chemical…
Q: One day in lab, while adding a gnarled root to a dark liquid bubbling in an iron cauldron, your…
A:
Q: 1. It is the ratio of Fe to H, when the equation below is balances Fe(s) + H,0(g)→Fe,O,(s)+H¿(g) a)…
A: Since you have asked multiple questions, we will solve the first question for you. If youwant any…
Q: Nitrogen dioxide forming from the reaction of nitrogen and oxygen in the atmosphere. II. The…
A: In the above question, some reactions are given.In a chemical reaction, reactants reacts to form a…
Q: Write a balanced equation for each of the following reactions.(You may have to guess at one or more…
A: a. The reaction for the above process is as follows: Al2Se3(s) + 6H+(aq) --------------->…
Q: 1) Balance all these equations. (Hint, only small numbers are needed) 2) Determine the theoretical…
A: Answer
Q: What is the oxidation state of iron (Fe) in the reactant Fe(s)? What is the oxidation state of…
A: The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation…
Q: What coefficients (in order from left to right) are needed to balance the following chemical…
A: Given, ___Fe2O3(s) + ___C(s) → ___Fe(s) + ___CO(g) Coefficients are needed to balance the above…
Q: 15.00 mL of a 0.20 M solution of sulfuric acid (H2SO4) is mixed with 20.00 mL of a 0.20 M solution…
A: Sulfuric acid react potassium hydroxide produced potassium sulphate salt and water
Step by step
Solved in 2 steps with 1 images

- Write a balanced equation for each of the following reactions.(You may have to guess at one or more of the reaction products,but you should be able to make a reasonable guess, basedon your study of this chapter.) (a) Hydrogen selenide can beprepared by reaction of an aqueous acid solution on aluminumselenide. (b) Sodium thiosulfate is used to remove excessCl2 from chlorine-bleached fabrics. The thiosulfate ion formsSO42- and elemental sulfur, while Cl2 is reduced to Cl - .Oxalic acid C2H2O4 is a metabolic product of many molds. Although oxalic acid is toxic to humans if ingested, many plants and vegetables contain significant amounts of oxalic acid or oxalate salts. In solution, oxalic acid can be oxidized by air via the following chemical equation: H2C2O4 (aq) + O2 (g) -> H2O2 (l) + 2 CO2 (g) If a plant metabolized enough oxalic acid to produce 3.2L of CO2 on a day when the temperature was 29°C and the pressure was 752 mmHg, a) how many grams of oxalic acid were converted to CO2? b) Given that air is 21% oxygen, what volume of air was needed for the oxidation?1. How many metric tons of charcoal is required to reduce 1500 kg of arsenic trioxide?AS2O3 + C = CO + As 2. The Le Blanc Process for the manufacture of soda ash encompasses the four steps:NaCl + H2SO4 = NaHSO4 + HClNaHSO4 + NaCl = Na2SO4 + HClNa2SO4 + C = Na2S + CO2Na2S + CaCO3 = Na2CO3 + CaSHow many tonnes of soda ash are produced per 5 tonnes of salt? How many kg of C are required?
- Magnesium nitrate and barium nitrate decompose similarly on heating and the reaction can be represented as follow: M(NO3)2 (s) --› MO (s) + 2NO2 (g) + ½O2 (g), where M = Mg or Ba (a) When Group 1 nitrates (with the exception of lithium nitrate) are strongly heated, the corresponding nitrite was formed along with a colorless gas which relights a glowing splint. (i) Write a balanced equation for the thermal decomposition of potassium nitrate. (ii) Unlike other Group 1 nitrates, lithium nitrate decomposes on heating in the same way as Group 2 nitrates. Suggest a reason for the difference in behaviour, and give an equation for its decomposition. (b) Zinc carbonate and magnesium carbonate decompose when heated to give similar products. (i) Suggest an equation for the thermal decomposition of magnesium carbonate. (ii) The radius of Zn2+ cation is 0.074 nm. Use the Data Booklet to deduce whether zinc carbonate will decompose at a higher or lower temperature than magnesium carbonate. Explain…The reaction between ammonia and nitrous oxide is given below:2 NH3(g) + 3 N2O(g) 4 N2(g) + 3 H2O(g)We therefore know that which of the following reactions can also occur? 5 N2(g) + 6 H2O(l) -> 4 NH3(g) + 6 NO(g) 4 N2(g) + 3 H2O(g) -> 2 NH3(g) + 3 N2O(g) 4 NH3(g) + 6 NO(g) -> 5 N2(g) + 6 H2O(l) None of the AboveBalance the reaction between Fe2+ and MnO4- to form Fe3+ and Mn2+ in acidic solution. When you have balanced the equation using the smallest integers possible, enter the coefficients of the species shown. Fe2+ + MnO4- Fe3+ + Mn2+Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)How many electrons are transferred in this reaction?
- We have talked about in lecture about metamorphic grade, and about the difference between progradeand retrograde mineral reactions. One such possible mineral reaction is as follows:orthoclase + sillimanite + H2O = muscovite + quartz 3a: Write a balanced chemical reaction for the above minerals, given that the formula for muscovite isK2Al6Si6O20(OH)4 3b: Indicate which reaction direction (left to right, right to left) is the prograde direction, and explainwhy you think that.4. Write a balanced equation for the following reaction: Tin (II) sulfide reacts with oxygen to form tin (II) oxide and sulfur dioxide. Group of answer choices a) 2SnS + 3O2 --> 2 SnO + 2SO2 b) TnS + 3O ----> TnO + SO2 c) 2SnS + O2 -----> 2SnO + S2O d) 2SnS + 4O ----->. 2SnO + S2OBalance the following equations and indicate whetherthey are combination, decomposition, or combustionreactions:(a) PbCO31s2¡PbO1s2 + CO21g2(b) C2H41g2 + O21g2¡CO21g2 + H2O1g2(c) Mg1s2 + N21g2¡Mg3N21s2(d) C7H8O21l2 + O21g2¡CO21g2 + H2O1g2(e) Al1s2 + Cl21g2¡AlCl31s2
- Direction: Balance the following chemical reactions. 7. COCl2 + H2O ->HCl + CO28. CS2 + O2 ->CO2 + SO29. H2SO4 + NaCN ->HCN + Na2SO4Heating MgNH4PO4 6H2O to 350ºC decomposes it to form ammonia, water, and magnesium pyrophosphate (Mg2P2O7). Balance this reaction.One day in lab, while taking apart a complicated distillation apparatus, your friend Maya (an expert chemist) says this: "Metal sulfides roasted with oxygen produce the corresponding oxide and sulfur dioxide gas." Using Maya's statement, and what you already know about chemistry, predict the products of the following reaction. Be sure your chemical equation is balanced! ZnS(s) +O2(g) ->



