15.) Determine the change in entropy of the reaction: SO2 (g) + H2O2 (1) → H2SO4 (1). The standard entropies for each species are 248.22, 188.83, 156.90 J/K mol for SO2 (g), H2O2 (1) and H2SO4 (1) respectively. 200 1rL
15.) Determine the change in entropy of the reaction: SO2 (g) + H2O2 (1) → H2SO4 (1). The standard entropies for each species are 248.22, 188.83, 156.90 J/K mol for SO2 (g), H2O2 (1) and H2SO4 (1) respectively. 200 1rL
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 93QRT: The standard molar entropy of iodine vapor, I2(g), is 260.7 J Kl mol-1 and the standard molar...
Related questions
Question
Please answer 15-18.
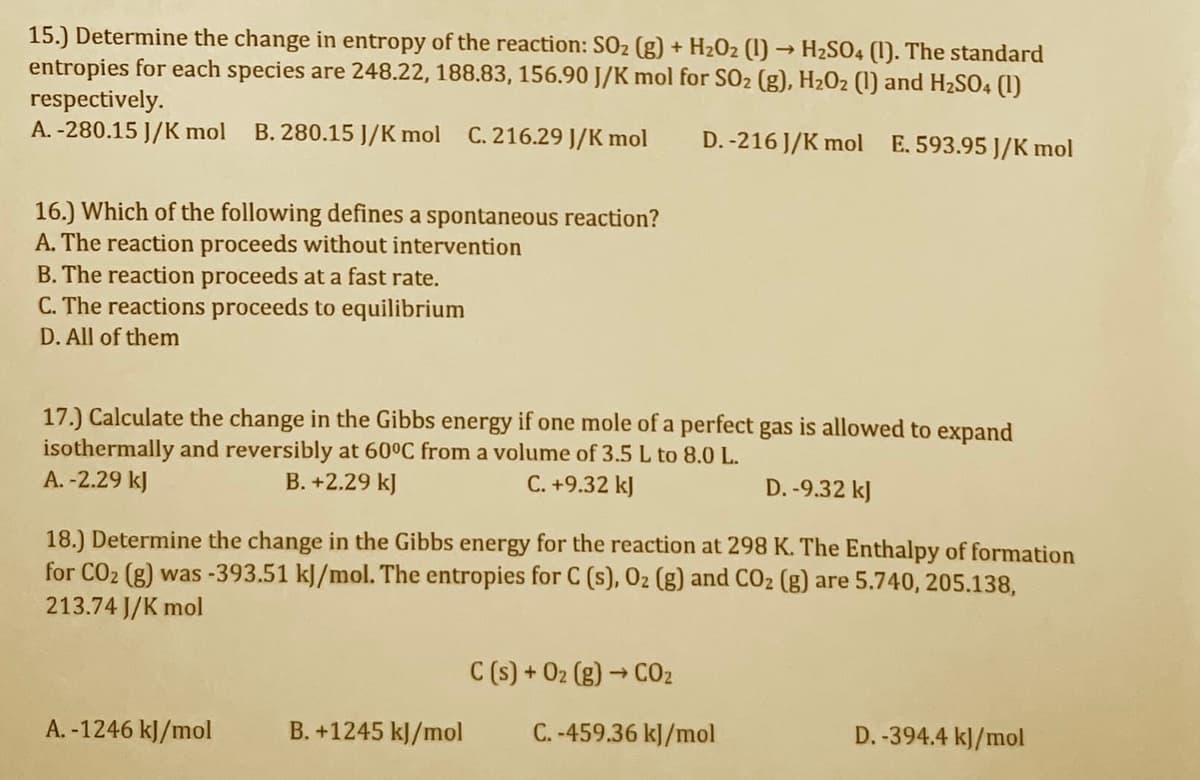
Transcribed Image Text:15.) Determine the change in entropy of the reaction: SO2 (g) + H2O2 (1) → H2SO4 (1). The standard
entropies for each species are 248.22, 188.83, 156.90 J/K mol for SO2 (g), H2O2 (1) and H2SO4 (1)
respectively.
A. -280.15 J/K mol
B. 280.15 J/K mol C. 216.29 J/K mol
D. -216 J/K mol E. 593.95 J/K mol
16.) Which of the following defines a spontaneous reaction?
A. The reaction proceeds without intervention
B. The reaction proceeds at a fast rate.
C. The reactions proceeds to equilibrium
D. All of them
17.) Calculate the change in the Gibbs energy if one mole of a perfect gas is allowed to expand
isothermally and reversibly at 60°C from a volume of 3.5 L to 8.0 L.
A. -2.29 kJ
B. +2.29 kJ
C. +9.32 kJ
D. -9.32 kJ
18.) Determine the change in the Gibbs energy for the reaction at 298 K. The Enthalpy of formation
for CO2 (g) was -393.51 kJ/mol. The entropies for C (s), 02 (g) and CO2 (g) are 5.740, 205.138,
213.74 J/K mol
C (s) + 02 (g) → C02
A. -1246 kJ/mol
B. +1245 kJ/mol
C. -459.36 kJ/mol
D. -394.4 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
