17. Consider the formation of the three solutions shown in the table. Rank the formation of the solutions from most endothermic to most exothermic. Strength of Interaction Soln. Type of interaction Soln. Soln. II III solute-solute weak strong strong weak strong weak solvent-solvent weak solute-solvent strong strong exothermicity → A. I< II< II В. I
17. Consider the formation of the three solutions shown in the table. Rank the formation of the solutions from most endothermic to most exothermic. Strength of Interaction Soln. Type of interaction Soln. Soln. II III solute-solute weak strong strong weak strong weak solvent-solvent weak solute-solvent strong strong exothermicity → A. I< II< II В. I
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 6RQ: In terms of Raoults law, distinguish between an ideal liquid-liquid solution and a nonideal...
Related questions
Question
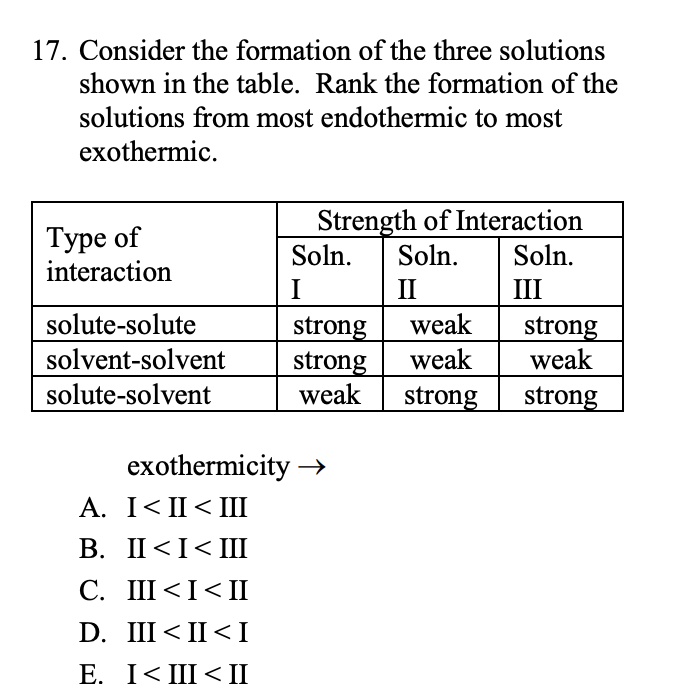
Transcribed Image Text:17. Consider the formation of the three solutions
shown in the table. Rank the formation of the
solutions from most endothermic to most
exothermic.
Strength of Interaction
Soln.
Type of
interaction
Soln.
Soln.
II
III
solute-solute
weak
strong
strong
weak
strong
weak
solvent-solvent
weak
solute-solvent
strong
strong
exothermicity →
A. I< II< II
В. I<I<Ш
C. III <I<II
D. III < II <I
E. I< III<II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT