17. Table 3 shows the first 8 ionisation energies of elements X and Y. * Talıle 3 Onder of clectron remaved lonisation eacrgy for XAJ mol lonisation encrgy for YAJ mol Ist 2nd 3ni 4th Sth 7th Sth 1000 2252 3363 4556 7004 8496 27108 31724 1314 3388 $301 7469 10990 13327 71330 84078 The proton mnmber of clements X aand Y is less than 20. Which of the following statentents is troe? A. X'forms an ionic chloride. B. The atomic radius of Xis smailer than Y. C. Xcombines with Y to form a lincar reolecule. D. Xand Y befong to the sume group in the Periodic Table.
17. Table 3 shows the first 8 ionisation energies of elements X and Y. * Talıle 3 Onder of clectron remaved lonisation eacrgy for XAJ mol lonisation encrgy for YAJ mol Ist 2nd 3ni 4th Sth 7th Sth 1000 2252 3363 4556 7004 8496 27108 31724 1314 3388 $301 7469 10990 13327 71330 84078 The proton mnmber of clements X aand Y is less than 20. Which of the following statentents is troe? A. X'forms an ionic chloride. B. The atomic radius of Xis smailer than Y. C. Xcombines with Y to form a lincar reolecule. D. Xand Y befong to the sume group in the Periodic Table.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 30E: The reaction 2I-(aq)+S2O82-(aq)I2(aq)+2SO42-(aq) was studied at 25C. The following results were...
Related questions
Question
100%
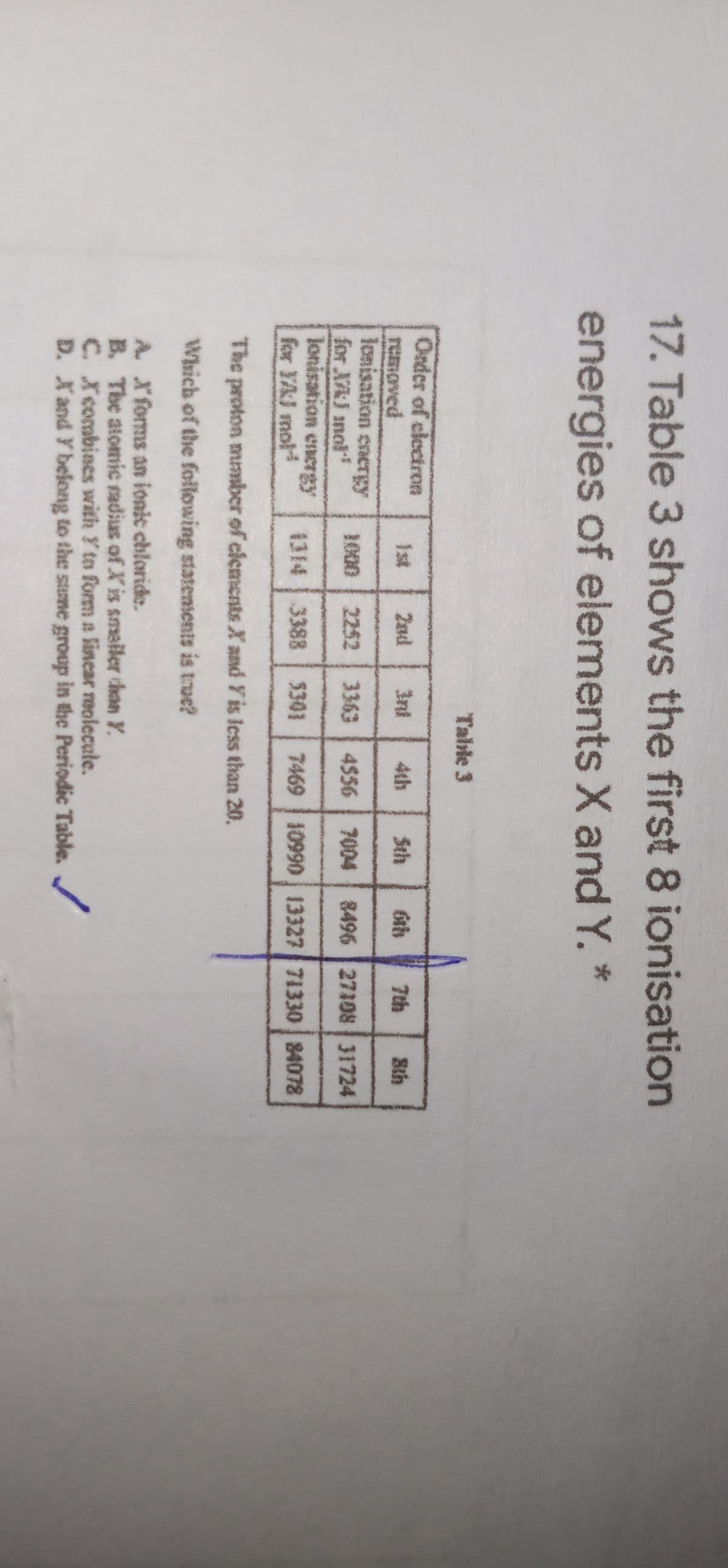
Transcribed Image Text:17. Table 3 shows the first 8 ionisation
energies of elements X and Y. *
Table 3
Onder of clectron
Ist
2ad
3ni
4th
Sth
7th
removed
lonisation energy
for XA) mol
Jonisation encrgy
for YAJ mol
1000
2252 3363 4556 7004
8496 27108 31724
3388 $301
7469 10990 13327 71330 84078
The proton mmmber of clements X and Y is less than 20.
Which of the foilowing statensents is true?
A Xforms an ionit chloride.
B. The alomic radius of Xis smaller than Y.
C. Xcombines with Y to form a lincar reolecule.
D. Xand Y belong to the sume group in the Periodic Table.
![16. The reaction between A and B is
%23
as follows: *
A + 2B 2C
The rate of reaction with variation of concentration of A and B at the same temperature is
shown in Table 2.
Table 2
Experiment [A]/mol dm [B/mol dm
2.00
1.50
1.00
0.50
1.00
1.00
1.50
2.00
Rate of reaction
Imol dm s
1.20 x 10
9.00 x 10
1.35 x 10
II
III
IV
What is the value of x?
3.00x 10
B..00 x 103
C. 1.20x 102
D. 2.40 x 102
A.3.00X 10-7
B.6.00 x10
C.1-20 X10-3
b- 240 x 10-3
you
20](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc26f0018-622b-4f92-b1c5-6920c8f26e23%2Fe717ce50-4589-41e5-8a2e-a69213e48aad%2Fheoaqa_processed.jpeg&w=3840&q=75)
Transcribed Image Text:16. The reaction between A and B is
%23
as follows: *
A + 2B 2C
The rate of reaction with variation of concentration of A and B at the same temperature is
shown in Table 2.
Table 2
Experiment [A]/mol dm [B/mol dm
2.00
1.50
1.00
0.50
1.00
1.00
1.50
2.00
Rate of reaction
Imol dm s
1.20 x 10
9.00 x 10
1.35 x 10
II
III
IV
What is the value of x?
3.00x 10
B..00 x 103
C. 1.20x 102
D. 2.40 x 102
A.3.00X 10-7
B.6.00 x10
C.1-20 X10-3
b- 240 x 10-3
you
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning