17.66 The "filmstrip" represents five molecular scenes of a gas- eous mixture as it reaches equilibrium over time: A в D E X is purple and Y is orange: X,(g) + Y,(g) = 2XY(g). (a) Write the reaction quotient, Q, for this reaction. (b) If each particle represents 0.1 mol, find Q for each scene. (c) If K> 1, is time progressing to the right or to the left? Explain. (d) Calculate K at this temperature. (e) If AHn < 0, which scene, if any, best represents the mixture at a higher temperature? Explain. (f) Which scene, if any, best represents the mixture at a higher pressure (lower volume)? Explain. rxn
17.66 The "filmstrip" represents five molecular scenes of a gas- eous mixture as it reaches equilibrium over time: A в D E X is purple and Y is orange: X,(g) + Y,(g) = 2XY(g). (a) Write the reaction quotient, Q, for this reaction. (b) If each particle represents 0.1 mol, find Q for each scene. (c) If K> 1, is time progressing to the right or to the left? Explain. (d) Calculate K at this temperature. (e) If AHn < 0, which scene, if any, best represents the mixture at a higher temperature? Explain. (f) Which scene, if any, best represents the mixture at a higher pressure (lower volume)? Explain. rxn
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.79PAE
Related questions
Question
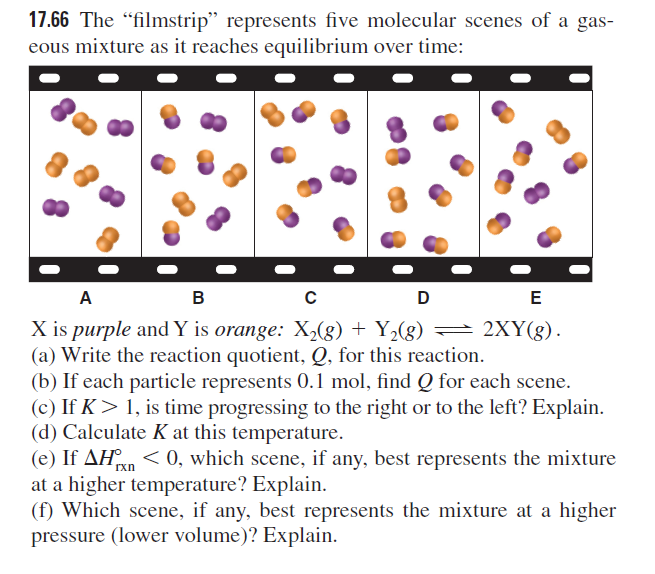
Transcribed Image Text:17.66 The "filmstrip" represents five molecular scenes of a gas-
eous mixture as it reaches equilibrium over time:
A
в
D
E
X is purple and Y is orange: X,(g) + Y,(g) = 2XY(g).
(a) Write the reaction quotient, Q, for this reaction.
(b) If each particle represents 0.1 mol, find Q for each scene.
(c) If K> 1, is time progressing to the right or to the left? Explain.
(d) Calculate K at this temperature.
(e) If AHn < 0, which scene, if any, best represents the mixture
at a higher temperature? Explain.
(f) Which scene, if any, best represents the mixture at a higher
pressure (lower volume)? Explain.
rxn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning