1-Propanol (P = 20.9 Torr at 25 °C) and 2-propanol (P2 = 45.2 Torr at 25 °C) form ideal solutions in all proportions. %3D Let a1 and x2 represent the mole fractions of 1-propanol and 2-propanol in a liquid mixture, respectively, and y1 and represent the mole fractions of each in the vapor phase. Y2 For a solution of these liquids with a1 0.310, calculate the composition of the vapor phase at 25 °C.
1-Propanol (P = 20.9 Torr at 25 °C) and 2-propanol (P2 = 45.2 Torr at 25 °C) form ideal solutions in all proportions. %3D Let a1 and x2 represent the mole fractions of 1-propanol and 2-propanol in a liquid mixture, respectively, and y1 and represent the mole fractions of each in the vapor phase. Y2 For a solution of these liquids with a1 0.310, calculate the composition of the vapor phase at 25 °C.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 114CP: Carbon tetrachloride (CCl4) and benzene (C6H6) form ideal solutions. Consider an equimolar solution...
Related questions
Question
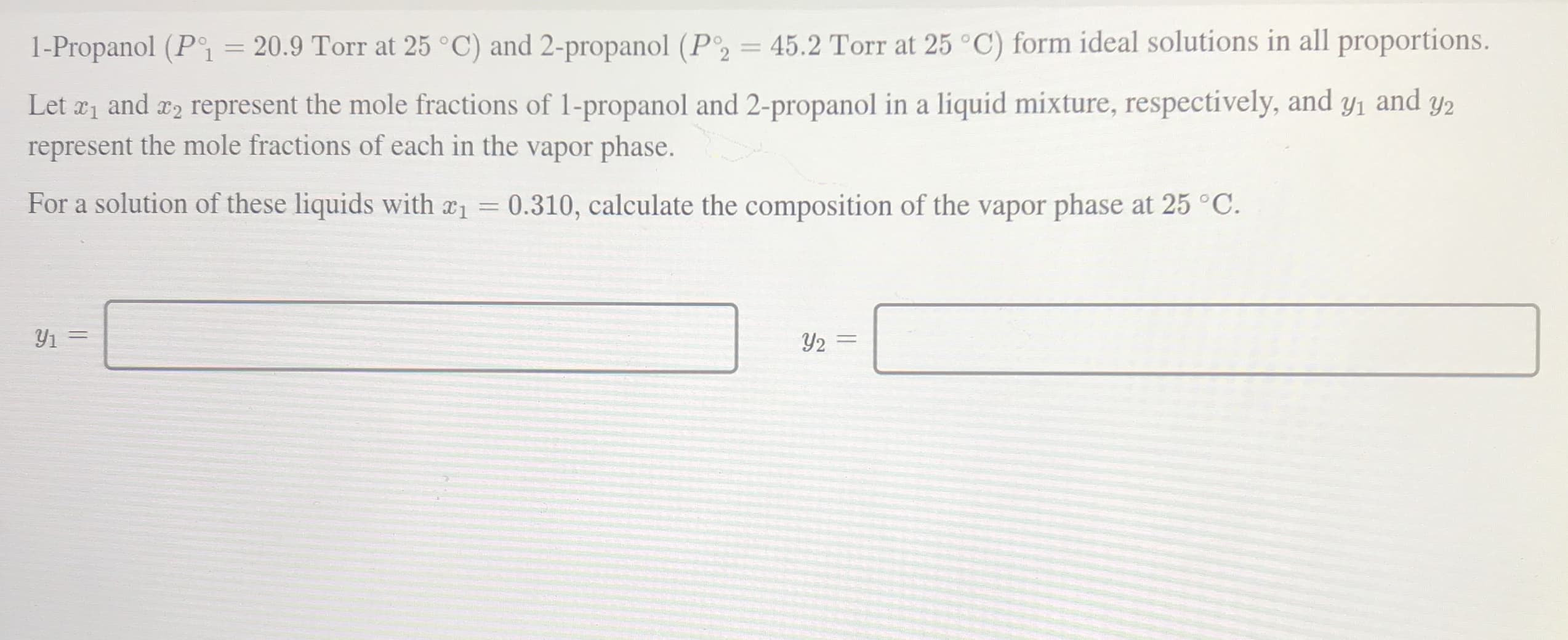
Transcribed Image Text:1-Propanol (P = 20.9 Torr at 25 °C) and 2-propanol (P2 = 45.2 Torr at 25 °C) form ideal solutions in all proportions.
%3D
Let a1 and x2 represent the mole fractions of 1-propanol and 2-propanol in a liquid mixture, respectively, and y1 and
represent the mole fractions of each in the vapor phase.
Y2
For a solution of these liquids with a1 0.310, calculate the composition of the vapor phase at 25 °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning