2 1/1 point For the reaction below, select all the correct statements: CH3OH(aq) + Cr2O72(aq) →CH2O(aq) +Cr3+(aq) The oxidation number of C in CH3OH is -2 Cr is oxidized in the reaction The oxidizing agent is Cr2O72- The reducing agent is CH2O
2 1/1 point For the reaction below, select all the correct statements: CH3OH(aq) + Cr2O72(aq) →CH2O(aq) +Cr3+(aq) The oxidation number of C in CH3OH is -2 Cr is oxidized in the reaction The oxidizing agent is Cr2O72- The reducing agent is CH2O
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter21: Chemistry Of The Main-group Elements
Section: Chapter Questions
Problem 21.163QP
Question
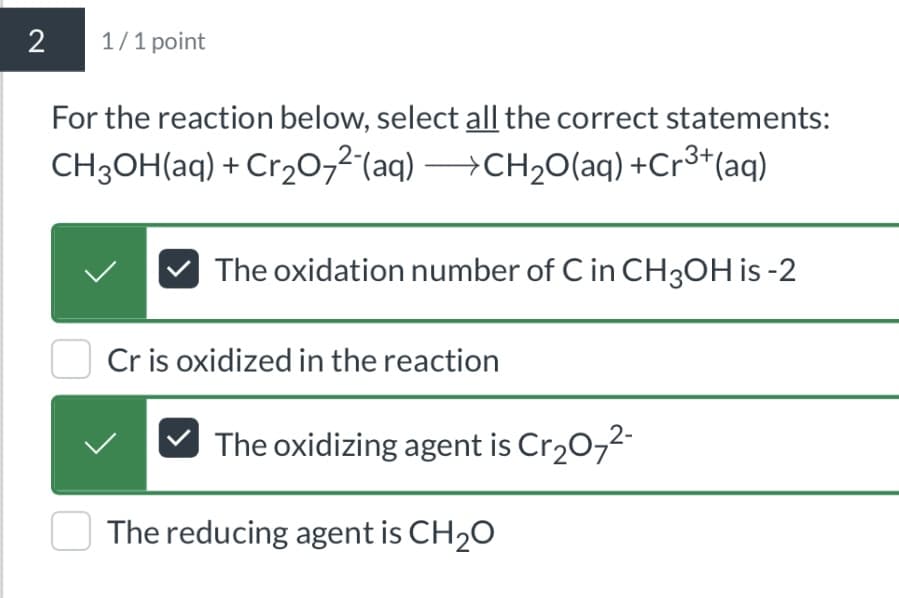
Transcribed Image Text:2
1/1 point
For the reaction below, select all the correct statements:
CH3OH(aq) + Cr2O72(aq) →CH2O(aq) +Cr3+(aq)
The oxidation number of C in CH3OH is -2
Cr is oxidized in the reaction
The oxidizing agent is Cr2O72-
The reducing agent is CH2O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning