2 CO(g) + O2(g) ----> 2 CO2 (g) Use the following conversions: 1 mol = MW in g (2 d.p.) = 22.414 L If 50.0 L of COe) (MW = 28.01g CO) reacts with 50.0 g of O2le) (MW = 32.00 g O2), what is the limiting reagent: CO or 02? 1._-- 3. 50.0 L CO x 2. 4. 5. 8. 9._ 10. 50.0 g 02 6. 7. .____- The limiting reagent is 11.
2 CO(g) + O2(g) ----> 2 CO2 (g) Use the following conversions: 1 mol = MW in g (2 d.p.) = 22.414 L If 50.0 L of COe) (MW = 28.01g CO) reacts with 50.0 g of O2le) (MW = 32.00 g O2), what is the limiting reagent: CO or 02? 1._-- 3. 50.0 L CO x 2. 4. 5. 8. 9._ 10. 50.0 g 02 6. 7. .____- The limiting reagent is 11.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.161QP
Related questions
Question
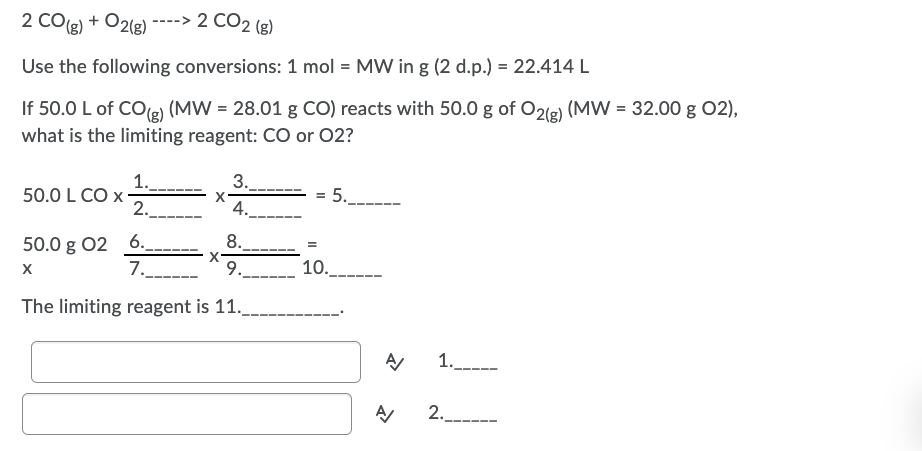
Transcribed Image Text:2 CO(g) + O2(8)
----> 2 CO2 (g)
Use the following conversions: 1 mol = MW in g (2 d.p.) = 22.414 L
If 50.0 L of COe) (MW = 28.01 g CO) reacts with 50.0 g of O21e) (MW = 32.00 g 02),
what is the limiting reagent: CO or 02?
1._.
50.0 L CO x
2.
3.
4.
= 5.
8.
50.0 g 02 6.
7.
9. 10._-
The limiting reagent is 11.
1.
2._-
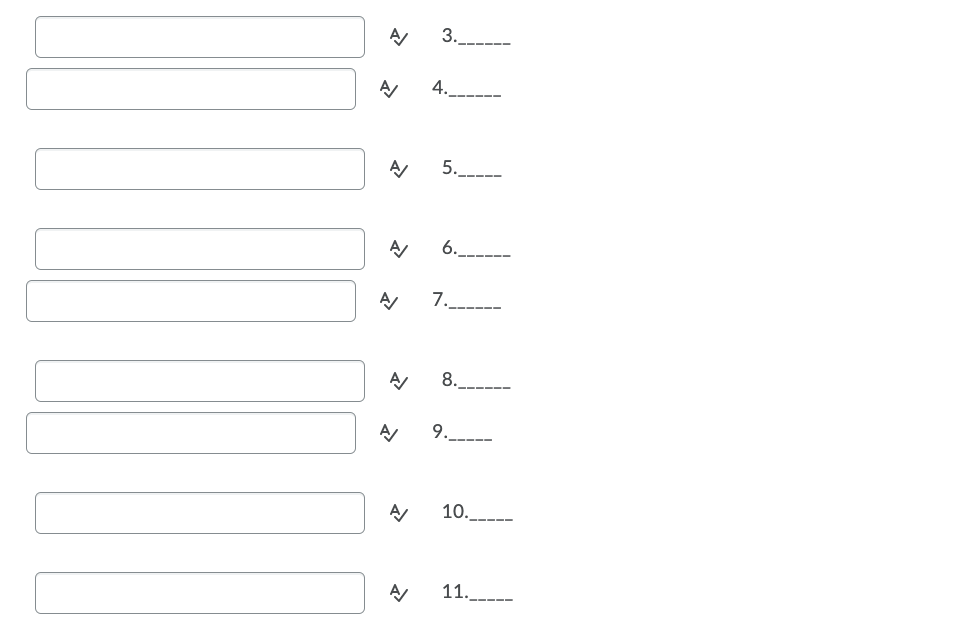
Transcribed Image Text:3.
4.
5.
シ
6.
7._-
8._-.
9._.
10.
11._.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning