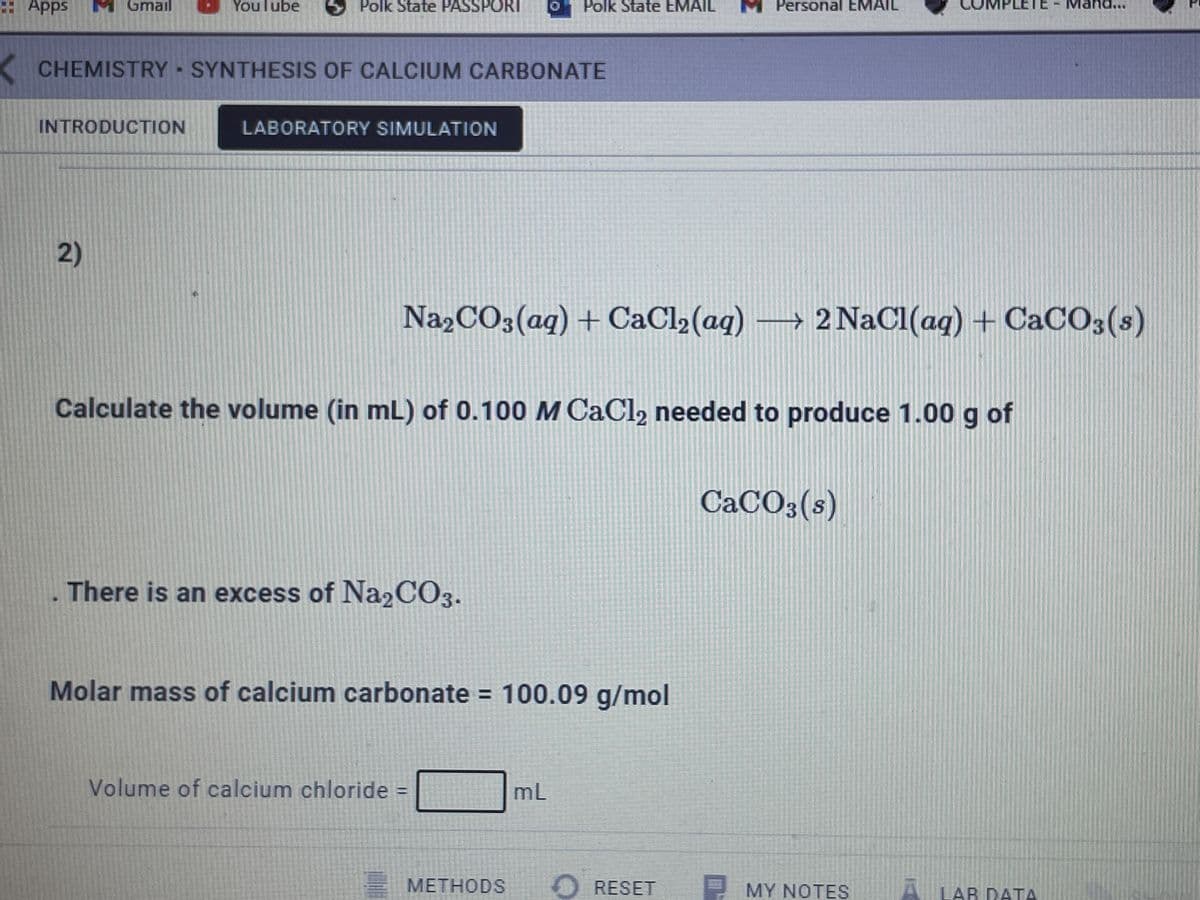
2) Na2CO3(aq) +CaCl₂(aq) → 2 NaCl(aq) + CaCO3(s) Calculate the volume (in mL) of 0.100 M CaCl₂ needed to produce 1.00 g of There is an excess of Na2CO3. Molar mass of calcium carbonate = 100.09 g/mol Volume of calcium chloride = mL CaCO3(s)
2) Na2CO3(aq) +CaCl₂(aq) → 2 NaCl(aq) + CaCO3(s) Calculate the volume (in mL) of 0.100 M CaCl₂ needed to produce 1.00 g of There is an excess of Na2CO3. Molar mass of calcium carbonate = 100.09 g/mol Volume of calcium chloride = mL CaCO3(s)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 120IL
Related questions
Question
What is the answer to this problem? I thought it was 100 mL but that came up as incorrect .
Transcribed Image Text:: Apps
Gmail
YouTube
Polk State PASSPORT
Polk State EMAIL
Personal EMAIL
..
CHEMISTRY SYNTHESIS OF CALCIUM CARBONATE
INTRODUCTION
LABORATORY SIMULATION
2)
Na»CO3(aq) + CaCl2 (aq) → 2 NaCl(aq) + CaCO3(s)
Calculate the volume (in mL) of 0.100 M CaCl2 needed to produce 1.00 g of
CaCO3(s)
There is an excess of Na2CO3.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of calcium chloride
mL
MЕТНODS
O RESET
MY NOTES
A LAR DATA
АВ DАТА
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning