2 NO2(g) + N2Oa(g) NO2(g) reacts to form N20a(g) according to the equation above. A pure sample of NO2(g) is placed into a rigid, evacuated 0.500 L container. The initial pressure of the NO2(g) is 960 torr. The temperature is held constant until the NO2(g) reaches equilibrium with its product. The figure below shows how the pressure of the system changes while reaching equilibrium. Pressure (torr) 960 760 Time 7. Which is the correct description of the partial pressures of N204 and NO2? (A) The partial pressures of both N2O4 and NO2 are increasing. (B) The partial pressures of both N204 and NO2 are decreasing. (C) The partial pressure of N204 is increasing twice as fast as that of NO2 is decreasing. (D) The partial pressure of NO2 is decreasing twice as fast as that of N204 is increasing.
2 NO2(g) + N2Oa(g) NO2(g) reacts to form N20a(g) according to the equation above. A pure sample of NO2(g) is placed into a rigid, evacuated 0.500 L container. The initial pressure of the NO2(g) is 960 torr. The temperature is held constant until the NO2(g) reaches equilibrium with its product. The figure below shows how the pressure of the system changes while reaching equilibrium. Pressure (torr) 960 760 Time 7. Which is the correct description of the partial pressures of N204 and NO2? (A) The partial pressures of both N2O4 and NO2 are increasing. (B) The partial pressures of both N204 and NO2 are decreasing. (C) The partial pressure of N204 is increasing twice as fast as that of NO2 is decreasing. (D) The partial pressure of NO2 is decreasing twice as fast as that of N204 is increasing.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 31Q
Related questions
Question
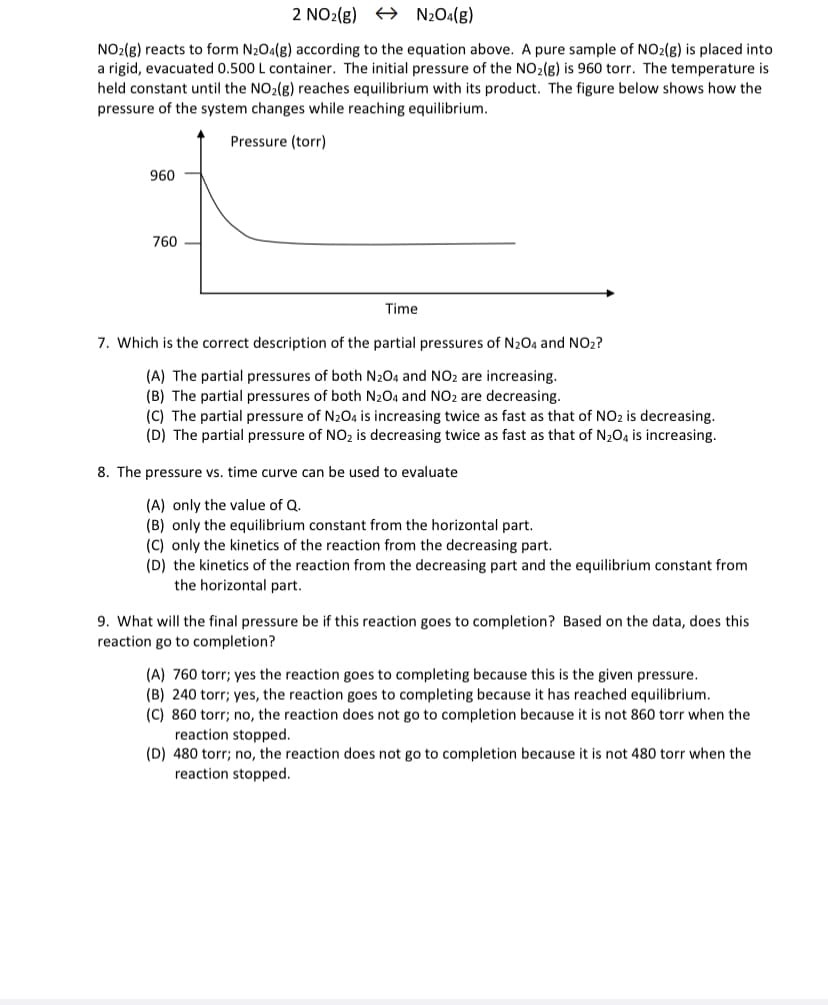
Transcribed Image Text:2 NO2(g) + N2O4(g)
NO2(g) reacts to form N204(g) according to the equation above. A pure sample of NO2(g) is placed into
a rigid, evacuated 0.500 L container. The initial pressure of the NO2(g) is 960 torr. The temperature is
held constant until the NO2(g) reaches equilibrium with its product. The figure below shows how the
pressure of the system changes while reaching equilibrium.
Pressure (torr)
960
760
Time
7. Which is the correct description of the partial pressures of N204 and NO2?
(A) The partial pressures of both N204 and NO2 are increasing.
(B) The partial pressures of both N204 and NO2 are decreasing.
(C) The partial pressure of N204 is increasing twice as fast as that of NO2 is decreasing.
(D) The partial pressure of NO, is decreasing twice as fast as that of N204 is increasing.
8. The pressure vs. time curve can be used to evaluate
(A) only the value of Q.
(B) only the equilibrium constant from the horizontal part.
(C) only the kinetics of the reaction from the decreasing part.
(D) the kinetics of the reaction from the decreasing part and the equilibrium constant from
the horizontal part.
9. What will the final pressure be if this reaction goes
completion? Based on the data, does this
reaction go to completion?
(A) 760 torr; yes the reaction goes to completing because this is the given pressure.
(B) 240 torr; yes, the reaction goes to completing because it has reached equilibrium.
(C) 860 torr; no, the reaction does not go to completion because it is not 860 torr when the
reaction stopped.
(D) 480 torr; no, the reaction does not go to completion because it is not 480 torr when the
reaction stopped.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning