2 of 2 The student then conducts an experiment to determine the solubility of AgCl in a 2.5 M NaCl solution at various temperatures. Some AgCl(s) remains in the container at all temperatures studied. The data are plotted on the graph below. 0.9 0.8- 0.7 0.6- 0.5- 0.4- 70 75 80 85 90 95 100 Temperature (°C) (h) A 1000. g sample of a saturated solution at 95 Cis cooled to 85 C. Determine the mass of AgCl(s) that would precipitate out of the solution. Mass of AgCl Dissolved (g/1000 g solution)
2 of 2 The student then conducts an experiment to determine the solubility of AgCl in a 2.5 M NaCl solution at various temperatures. Some AgCl(s) remains in the container at all temperatures studied. The data are plotted on the graph below. 0.9 0.8- 0.7 0.6- 0.5- 0.4- 70 75 80 85 90 95 100 Temperature (°C) (h) A 1000. g sample of a saturated solution at 95 Cis cooled to 85 C. Determine the mass of AgCl(s) that would precipitate out of the solution. Mass of AgCl Dissolved (g/1000 g solution)
Chapter11: Health Of Aquatic Animals
Section: Chapter Questions
Problem 3KA
Related questions
Question
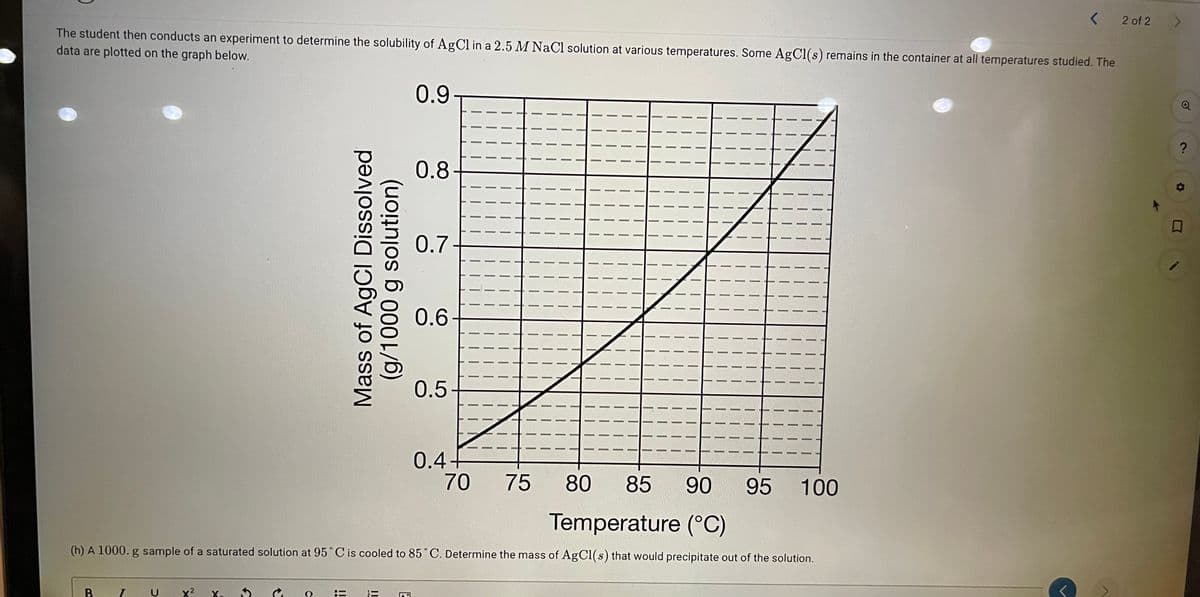
Transcribed Image Text:2 of 2
The student then conducts an experiment to determine the solubility of AgCl in a 2.5 M NaCl solution at various temperatures. Some AgCl(s) remains in the container at all temperatures studied. The
data are plotted on the graph below.
0.9
0.8
0.7
0.6
0.5-
0.4+
70
75
80
85
90
95 100
Temperature (°C)
(h) A 1000. g sample of a saturated solution at 95° C is cooled to 85°C. Determine the mass of AgCl(s) that would precipitate out of the solution.
B
U
x2
X.
Mass of AgCl Dissolved
solution)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



