2) Which answer choice represents the lowest whole number coefficients when the following equation is balanced? N2H4 + H202 –→N2 + H2O A) 1, 4, 2, 8 B) 1, 2, 1, 4 C) 2, 4, 2, 4 D) 2, 3, 2, 3
Q: When the equation below is balanced, what is the coefficient in front of the oxygen? __ C4H8S2 +…
A: To solve this first we have to balanced the chemical equation . The equation will be balanced when…
Q: how many grams of CO2 can be formed?
A:
Q: Calculate the % CO2 considering the reaction of 2.500 g of KHCO3 with excess HCl. The reaction is…
A: The % CO2 produced is to be calculated considering the reaction of 2.500 g of KHCO3 with excess HCl.…
Q: NH3 reacts with O2 to produce NO and H2O NH3+O2-->NO+H2O What is limiting reactant? a.) H2O…
A:
Q: Question 16 Consider the balanced equation shown below. How many moles of C would be required to…
A:
Q: for each of the following unbalanced equations, indicate how many moles of the first product are…
A:
Q: 5Cl-+ 2MnO4- + 11 H+ →
A: Dear student I have given answer to your question in the image format.
Q: HELP me please
A:
Q: What is the coefficient of H2O when the following equation is balanced with the smallest set of…
A: According to the Law of conservation of mass " all atoms of different elements must be equal on both…
Q: What is the coefficient in front of oxygen gas when the following chemical equation is balanced?…
A: Given, Chemical reaction : C3H6(g) + O2(g) ⟶⟶ CO2(g) + H2O(l)
Q: the quantities below were used for Step 1, the synthesis of FeC2O4•2H2O, which reagent would be the…
A: Given, mass of (NH4)2[Fe(H2O)6](SO4)2= 2.25 g mass of H2C2O4•2H2O =1.43 g
Q: Use the References to access important values if needed for this question. When the following…
A: Balance the chemical reaction using smallest possible integer coefficient
Q: D Question 31 N₂ (g) Which is the sum of the coefficients when the reaction below is balanced using…
A:
Q: 3. Is the following equation balanced? Explain your answer. N2 + H, NH3 -> Answer:
A: BALANCED REACTION The balance reaction for the given reaction is given below- N2 + 3H2 ----> 2NH3…
Q: Butane can be used as a fuel. How many moles of oxygen are needed to completely react with 3.0 moles…
A: given, moles of butane = 3.0 mol we need to calculate the moles of oxygen for complete reaction…
Q: What is the coefficient for O2 when this equation is balanced with the lowest whole number…
A: ⊙Given equation is :- C3H7OH + O2 →CO2+H2O —IAbove equation is the combination…
Q: What are the stoichiometric coefficients in the followingequation when it is balanced?CH4(g) +…
A: Correct answer is (e) 1, 2, 4, 1 Balanced chemical equation: CH4(g) + 2H2O(g) ⇌ 4H2(g) +…
Q: What are the possible stoichiometric factors for CH4+2O2---> CO2+2H2O
A:
Q: Al + Zn (CIoslz= Al (clos)s+ Zn Zn (cIos)2 = 1.270. Al = 0.9550g %3D %3D %3D QUESTION: WHAT IS THE…
A:
Q: C3Hg(g)+___O2(g)-->3CO2(g)+4H2O(l) How many O atoms are present on the right side of the chemical…
A: The given chemical equation is, C3H8 (g) + ___O2(g) → 3CO2(g) + 4 H2O(l)
Q: Which of the following equations is not balanced? Group of answer choices C3H8 + 5 O2 3 CO2 + 4 H2O…
A:
Q: Find the limiting reactant ____ C5H9O + ____ O2 -----> ____ CO2 + ____ H2O
A: Combustion reaction can be described as a chemical equation where a substance reacts with the excess…
Q: Balance the following equation and then solve the question below. e2O3 +SO3 → Fe2(SO4)3 alculate the…
A:
Q: If 0.00516 mols of HC2H3O2 are reacted with 0.00585 mols of NaHCO3, which is the limiting reactant?…
A: The reactions taking place are, 1) NaHCO3 (s) + HC2H3O2 (aq) --------> C2H3O2Na (aq) + H2CO3 (aq)…
Q: Learning Check Balance each equation. А. Mg(s) +_N2(g) M93N2(s) RY В. Al(s) +_Cl2(g) _AlCl3(s) С.…
A: Note: Since you have posted a question with multiple subparts, we will solve the first three…
Q: 4. Given the reaction 2KCIO, à 2KCI+ 30,: 3 How many grams of O, will you get if 38g of KCIO,…
A: Given that, Mass of KClO3 = 38 gms Molar mass of KClO3 = 122.55 gm/mol So, Number of moles=Given…
Q: Balance the below chemical reaction. Ec Hg + O2 → EcO2 + H20 In the correctly balanced equation,…
A: Balanced chemical equation can be defined as the equation in which equal number of atoms of each…
Q: What is the limiting reagent when 2 moles of Al are reacted with 2 moles of O(subscript #2)? About…
A:
Q: Determine the limiting reactants (LR) and the mass (in grams) of nitrogen?
A: First, the given reactants mass are converted into moles as follows, The balanced equation for…
Q: a. Calculate the percent yield of CaC;0,H¿O using your actual yield yield calculated above.…
A: We have to find the percentage yield of reaction from given data, by finding limiting reagent and…
Q: When the equation below is balanced, what is the coefficient of oxygen gas? C7H16(g) + O2(g) -->…
A: Calculation of coefficient of O2 in balanced chemical equation.
Q: What is the sum of the coefficients after the following equation is balanced? Al203 + H2SO4 –→…
A: We can balance the reaction so that the reaction obey the law of conservation of mass. The number…
Q: C,H,0 + 30, → 2C0, + 3H,0 How many of each atom is present in the products? C= H=
A: The answer to the following question is given as -
Q: What is the sum of coefficients when the following reaction is balanced? __ Li + ___ N2 à…
A: Given :- __ Li + ___ N2 ----> ___Li3N To be determined :- Sum of coefficients in the…
Q: For this reaction, 2 NH3 + CO2 --> H2NCONH2 + H2O how many moles of CO2 are required to produce 3.0…
A: The balanced equation is: 2 NH3 + CO2 --> H2NCONH2 + H2O It is shown that 2 mol of NH3 react…
Q: (b) Calculate the percentage yield when 2.30 g of ethanol rencts completely with excess ethanoic…
A: Given that, 2.30 g of ethanol reacts completely with excess ethanoic acid to produce 3.52 g of ethyl…
Q: What are the coefficients when the following equation is properly balanced? 2H6S + O2 --> CO2 + SO2…
A: What are the coefficients of the given reaction--
Q: Which one or more of the following equations is (are) balanced? (Select all that apply.) O C7H16 +…
A:
Q: What are the correct coefficients for the following equation when balanced correctly?What are the…
A:
Q: Given 2 CL2 + Ti ---> TiCl4, how many moles of products will be produced from 1,205.3 grams of…
A:
Q: Consider the reaction 4Fe(s) + 302(g) --> 2FE203(s) If you started with and consumed 4 moles of O2,…
A:
Q: NH3 (g) + HCl(g) ---------> NH4Cl (s) How many moles of ammonium chloride would be produced by…
A: Given, The reaction is; NH3g + HClg→NH4Cls ......(1) Where, NH3 (g) = Ammonia HCl (g) =…
Q: 10. When the equation below is balanced, the coefficient in front of carbon dioxide is C3H8 + O2 -->…
A:
Q: Moving to another question will save this response. Question 15 properly balanced? Which one of the…
A: The first, third and fifth equations consist of unequal numbers of oxygen on both sides. The fourth…
Q: 2H2 + O2 ⟶⟶ 2H2O How many moles of O2 are needed to produce 10 moles of H2O?
A:
Q: Which is the limiting reactant in the following reaction given that you start with 4205 of CO 2 and…
A: Given reaction- CO2+2KOH -> K2CO3+H2O
Q: Due: Toda All changes saved 2. The combustion of glucose is represented by the following balanced…
A: Here limiting reactant is oxygen because it is completely consumed in the Reaction but C6H12O6 is…
Q: For equation 4NaCl + 2SO2 + _____H2O + _____O2 → _____Na2SO4 + 4HCl Which numbers balance this…
A: We need to balance the number of atoms of each element on left and right side of equation.
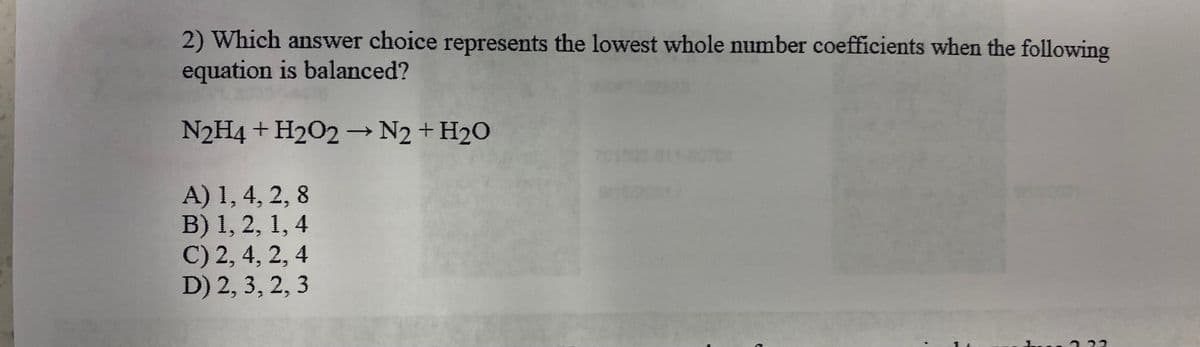
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Provided the following information determine Δ?ΔHformation of C2H6(g). C(graphite) + O2(g) ⟶⟶ CO2(g) Δ?=−393.5??ΔH=−393.5kJ H2(g) + 1212O2(g) ⟶⟶ H2O(l) Δ?=−285.8??ΔH=−285.8kJ C2H6(g) + 7272O2(g) ⟶⟶ 2CO2(g) + 3H2O(l) Δ?=−1560.7??Calculate the ΔG°rxn using the following information. 2 H2S(g) + 3 O2(g) → 2 SO2(g) + 2 H2O(g) ΔG°rxn = ? ΔG°f (kJ/mol) -33.4 -300.1 -228.6 Group of answer choices +990.6 kJ -1124 kJ +562.1 kJ +72.0 kJ -990.6 kJWhat is the Ksp expression for the following reaction? Bi2S3(s) ⇄ 2 Bi3+(aq) + 3 S2−(aq) Group of answer choices Ksp = [Bi3+]2[S2−]3 Ksp = [Bi3+]2[S2−]3/[Bi2S3] Ksp = [Bi3+]3[S2−]2 Ksp = [Bi2S3]/[Bi3+][S2−] Ksp = [Bi3+][S2−] PreviousNext
- ) Calculate the ΔG°rxn using the following information. 4 HNO3(g) + 5 N2H4(l) → 7 N2(g) + 12 H2O(l) ΔG°rxn = ? ΔG°f (kJ/mol) -73.5 149.3 -237.1From the enthalpies of reaction2 C1s2 + O21g2¡ 2 CO1g2 ΔH = -221.0 kJ2 C1s2 + O21g2 + 4 H21g2¡ 2 CH3OH1g2 ΔH = -402.4 kJcalculate ΔH for the reactionCO1g2 + 2 H21g2¡CH3OH1g2According to the conventions above, what is the sign ( + or ) of the P.E. change (H) for Rxn 3?
- please solve Q12) The primary standard must satisfy one of the following criteria a) Available in pure form b) Unstable c) hygroscopic d) Has Low molecular weightWhich of the reactions have a positive ΔSrxn? Select one or more: a. 2 A(g) + 2 B(g) ⟶ 5 C(g) b. A(s) + 2 B(g) ⟶ C(g) c. 2 A(g) + 3 B(g) ⟶ 4 C(g) d. A(s) + B(g) ⟶ 2 C(g)Calculate the ΔG°rxn using the following information. 3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g) ΔG°f (kJ/mol) 51.3 −237.1 −110.9 87.6 Group of answer choices −51.0 kJ −87.6 kJ +162.5 kJ −162.5 kJ +51.0 kJ
- Calculate the ΔH°rxn for the following reaction. SiO2(s) + 4HCl(g) → SiCl4(g) + 2H2O(g) ΔH°f [SiO2(s)] = –910.9 kJ/mol; ΔH°f [SiCl4(g)] = –657.0 kJ/mol; ΔH°f [HCl(g)] = –92.3 kJ/mol; ΔH°f [H2O(g)] = –241.8 kJ/mol Group of answer choices –139.5 kJ –137.4 kJ –104.4 kJ 104.4 kJ 139.5 kJ1. Consider the following eqm: SO2(g) + NO2(g) ⇌ NO(g) + SO3(g)What effect will adding SO3 have on the rxn?What effect will removing NO2 have on the rxn? 2. Consider the following exothermic rxn at eqm: C3H8(g) + 5O2(g) ⇌ 4H2O(l) + 3CO2(g)What effect will increasing the temperature have on the rxn?What effect will increasing the pressure have on the rxn?Find the deltaH for the reaction below, given the following reactions and subsequent deltaH values: N2H4(l) + H2(g) —> 2NH3 (g) N2H4(l) + CH4O(l) —> CH2O (g) + N2(g) + 3H2(g)—— deltaH= -32.8kj N2(g)+ 3H2(g) —> 2NH3 (g)—deltaH=-57.2 kj CH4O(l) —>CH2O(g) + H2(g) —-deltaH= -65 kj SIG FIGS

