2) You are tasked with determining the barium content of a sample containing barium nitrate mixed with rubidium nitrate. The sample is weighed, dissolved into 50.0mg water, and treated with xs 0.500M sodium sulfate. The acid fully ionizes in this experiment. A white precipitate forms which is washed, filtered, and weighed multiple times, according to the data given below: Mass of sample 0.425g Mass of thoroughly dried filter paper 1.462g Mass of precipitate+filter after 1st drying 1.755g Mass of precipitate+filter after 2nd drying 1.699g Mass of precipitate+filter after 3rd drying 1.698g C] In the filtrate solution, is [Na'] less than, equal to, or greater than the [NO,] level? Justify your answer.
2) You are tasked with determining the barium content of a sample containing barium nitrate mixed with rubidium nitrate. The sample is weighed, dissolved into 50.0mg water, and treated with xs 0.500M sodium sulfate. The acid fully ionizes in this experiment. A white precipitate forms which is washed, filtered, and weighed multiple times, according to the data given below: Mass of sample 0.425g Mass of thoroughly dried filter paper 1.462g Mass of precipitate+filter after 1st drying 1.755g Mass of precipitate+filter after 2nd drying 1.699g Mass of precipitate+filter after 3rd drying 1.698g C] In the filtrate solution, is [Na'] less than, equal to, or greater than the [NO,] level? Justify your answer.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 65QAP: Twenty-five milliliters of a solution (d=1.107g/mL)containing 15.25% by mass of sulfuric acid is...
Related questions
Question
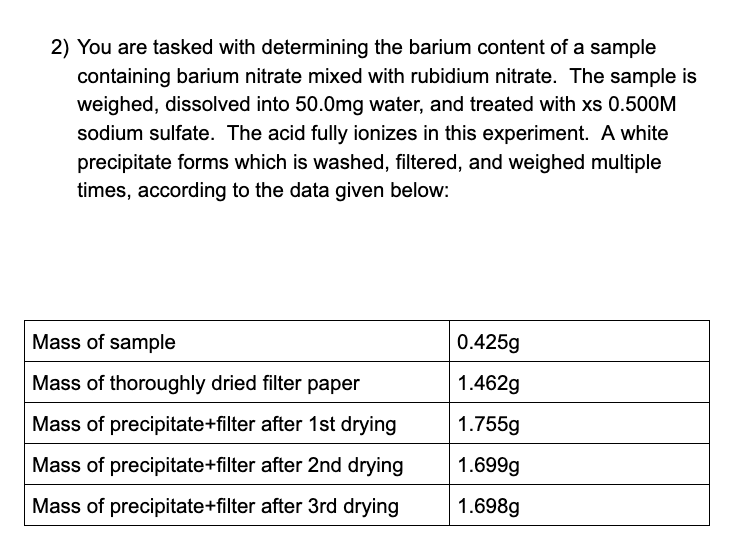
Transcribed Image Text:2) You are tasked with determining the barium content of a sample
containing barium nitrate mixed with rubidium nitrate. The sample is
weighed, dissolved into 50.0mg water, and treated with xs 0.500M
sodium sulfate. The acid fully ionizes in this experiment. A white
precipitate forms which is washed, filtered, and weighed multiple
times, according to the data given below:
Mass of sample
0.425g
Mass of thoroughly dried filter paper
1.462g
Mass of precipitate+filter after 1st drying
1.755g
Mass of precipitate+filter after 2nd drying
1.699g
Mass of precipitate+filter after 3rd drying
1.698g
![C] In the filtrate solution, is [Na'] less than, equal to, or greater than
the [NO,] level? Justify your answer.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ffa79d523-50fd-45b4-9804-70692af14db2%2Fa5f3f4f3-77e6-4664-9702-0e5ea17811c4%2Fjg8xago.png&w=3840&q=75)
Transcribed Image Text:C] In the filtrate solution, is [Na'] less than, equal to, or greater than
the [NO,] level? Justify your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning