2. Assume the following reaction has a 100% yield: 2Al(s) + 6HC10,(aq) 2Al(CIO,),(aq) + 3H,(g) > a) How many moles of HC1O, must react to produce 0.400 moles of H,? b) How many grams of HC10, are required to completely react with 50.0 g of Al? c) If 1.40 moles of H, are produced, how many grams of Al(CIO), would also be produced? emole negyxooo d) If 25.0 g of Al are mixed with a solution containing 150 g of HCIO, how many grams of H, could be produced? 49 e) (See part d) How many grams of which reactant is in excess?
2. Assume the following reaction has a 100% yield: 2Al(s) + 6HC10,(aq) 2Al(CIO,),(aq) + 3H,(g) > a) How many moles of HC1O, must react to produce 0.400 moles of H,? b) How many grams of HC10, are required to completely react with 50.0 g of Al? c) If 1.40 moles of H, are produced, how many grams of Al(CIO), would also be produced? emole negyxooo d) If 25.0 g of Al are mixed with a solution containing 150 g of HCIO, how many grams of H, could be produced? 49 e) (See part d) How many grams of which reactant is in excess?
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 57A
Related questions
Question
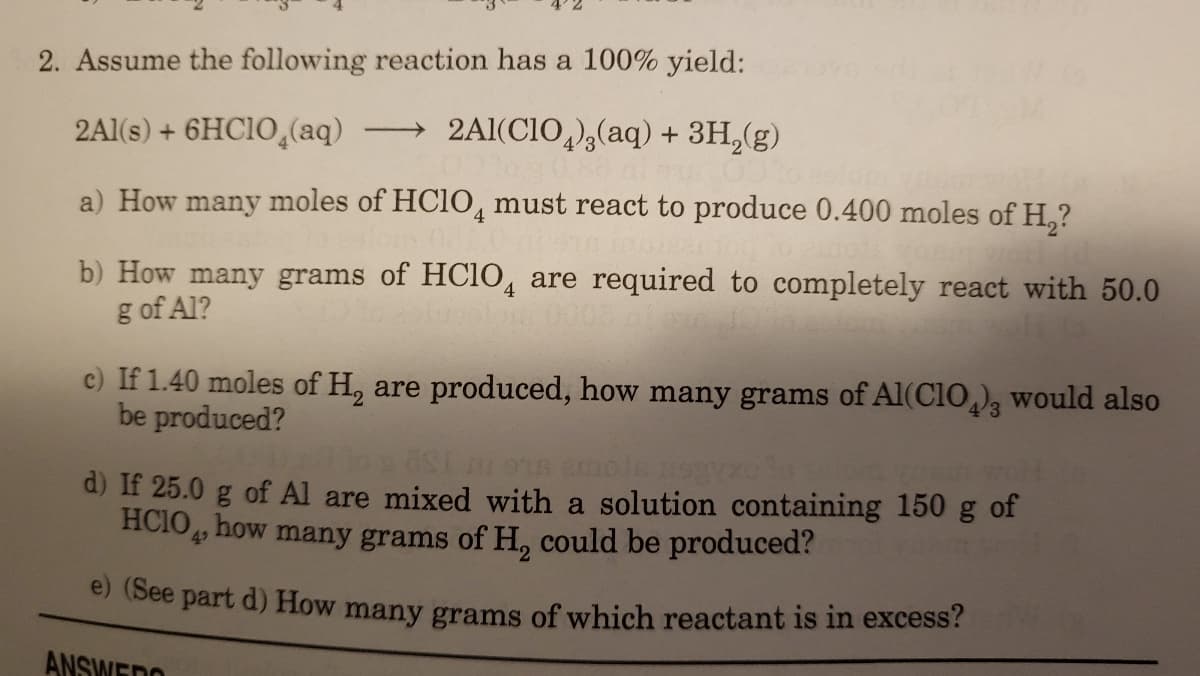
Transcribed Image Text:2. Assume the following reaction has a 100% yield:
2Al(s) + 6HC1O,(aq)
2Al(CIO,)3(aq) + 3H,(g)
|
a) How many moles of HC1O, must react to produce 0.400 moles of H,?
b) How many grams of HC1O, are required to completely react with 50.0
g of Al?
c) If 1.40 moles of H, are produced, how many grams of Al(CIO,), would also
be produced?
oSt ms emole negy
of Al are mixed with a solution containing 150 g of
d) If 25.0
HCIO, how many grams of H, could be produced?
49
e (See part d) How many grams of which reactant is in excess?
ANSWEDO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning