2. Ate Shawol and Concertina performed electrolysis to determine the identity of an unknown metal X. They determined that 3 electrons are involved in the reaction per mole of the unknown metal X. After applying 1.495 A of current for 18.00 hours, 17.41 g of the unknown metal was deposited to the electrode. Help Ate Shawol and Concertina determine the identity of the unknown metal X.
2. Ate Shawol and Concertina performed electrolysis to determine the identity of an unknown metal X. They determined that 3 electrons are involved in the reaction per mole of the unknown metal X. After applying 1.495 A of current for 18.00 hours, 17.41 g of the unknown metal was deposited to the electrode. Help Ate Shawol and Concertina determine the identity of the unknown metal X.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.145QP
Related questions
Question
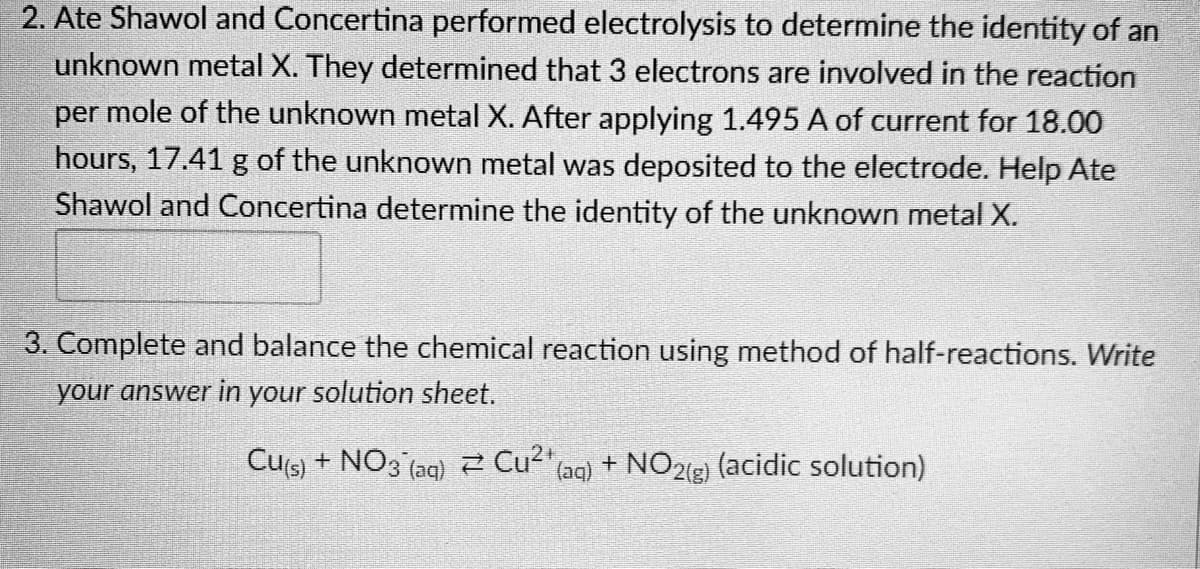
Transcribed Image Text:2. Ate Shawol and Concertina performed electrolysis to determine the identity of an
unknown metal X. They determined that 3 electrons are involved in the reaction
per mole of the unknown metal X. After applying 1.495 A of current for 18.00
hours, 17.41 g of the unknown metal was deposited to the electrode. Help Ate
Shawol and Concertina determine the identity of the unknown metal X.
3. Complete and balance the chemical reaction using method of half-reactions. Write
your answer in your solution sheet.
Cu(s) + NO3(aq) Cu²¹ (aq) + NO₂(g) (acidic solution)
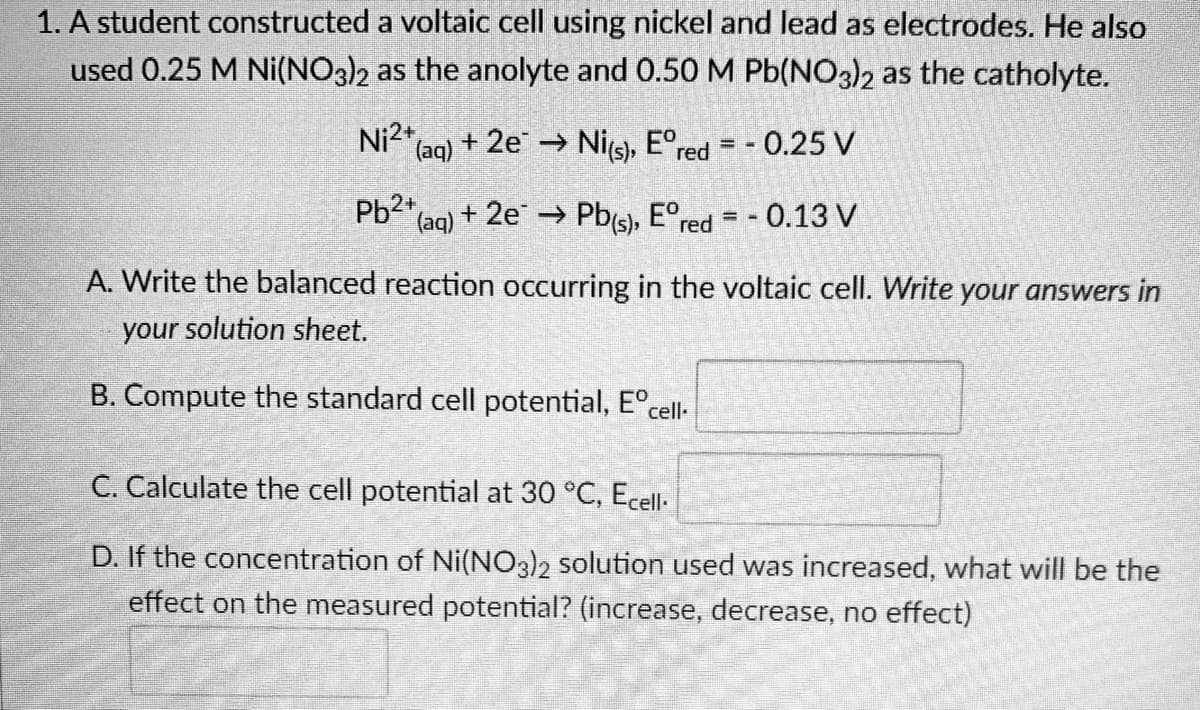
Transcribed Image Text:1. A student constructed
used 0.25 M Ni(NO3)2
a voltaic cell using nickel and lead as electrodes. He also
as the anolyte and 0.50 M Pb(NO3)2 as the catholyte.
Ni2+ (aq) + 2e → Ni(s), Eºred = -0.25 V
Pb2+ (aq) + 2e → Pb(s), Eºr - 0.13 V
red = -
A. Write the balanced reaction occurring in the voltaic cell. Write your answers in
your solution sheet.
B. Compute the standard cell potential, Eºcell-
C. Calculate the cell potential at 30 °C, Ecell-
D. If the concentration of Ni(NO3)2 solution used was increased, what will be the
effect on the measured potential? (increase, decrease, no effect)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning