
You have 1.0 M solutions of Al(NO3)3 and AgNO3 along with Al and Ag electrodes to construct a voltaic cell. The salt bridge contains a saturated solution of KCl. Complete the picture associated with this problem by
- a writing the
symbols of the elements and ions in the appropriate areas (both solutions and electrodes). - b identifying the anode and cathode.
- c indicating the direction of electron flow through the external circuit.
- d indicating the cell potential (assume standard conditions, with no current flowing).
- e writing the appropriate half-reaction under each of the containers.
- f indicating the direction of ion flow in the salt bridge.
- g identifying the species undergoing oxidation and reduction.
- h writing the balanced overall reaction for the cell.
(a)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
The symbols of elements and ions in cell are,
Symbols of Aluminium is Al, Silver is Ag, Aluminium ion is
Explanation of Solution
The symbols of elements and ions in cell are,
Symbol of Aluminium is Al and it is an anode
Symbol of Silver is Ag and it is an cathode
Symbol of Aluminium ion is
Symbol of Silver ion is
(b)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
Silver rod in Silver nitrate is a cathode and Aluminium rod in Aluminium nitrate is an anode.
Explanation of Solution
In given cell, Silver rod in Silver nitrate is a cathode and Aluminium rod in Aluminium nitrate is an anode.
(c)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
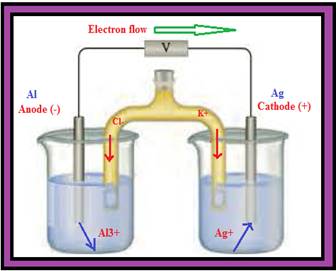
Figure 1
Explanation of Solution
(d)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
The cell potential (EMF) of given voltaic cell is
Explanation of Solution
The standard reduction potentials of (SRQ) of half cell reactions are record from standard reduction potentials table and they are,
The most positive SQR is considering as cathode potential.
The SQR of electrodes are plugged in the bellow equation to give cell potential of given voltaic cell.
The cell potential (EMF) of given voltaic cell is
(e)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
The Oxidation half cell reaction is,
The reduction half cell reaction is,
Explanation of Solution
The Oxidation half cell reaction is,
The reduction half cell reaction is,
(f)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
Explanation of Solution
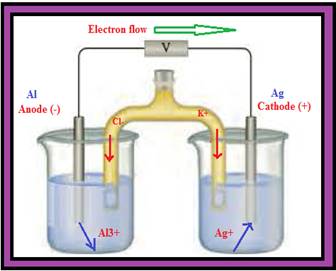
Figure 1
(g)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
Explanation of Solution
The Oxidation half cell reaction is,
The reduction half cell reaction is,
Hence,
(h)
Interpretation:
Anode, cathode, direction of electron flow, symbols of elements and ions in cell, EMF of the cell and half cell and balanced overall cell reactions should be given.
Concept introduction:
Voltaic cell:
The device, which is converting the chemical energy into electrical energy, is called voltaic cell and this conversation is takes place by the redox reaction.
The oxidation half reaction takes place in anode and reduction half reaction takes place in cathode.
From the result of this redox reaction the electron flow is form anode to cathode direction in outer circuit.
Cell potential (EMF):
The maximum potential difference between two electrodes of voltaic cell is known as cell potential.
If standard reduction potentials of electrodes are given the cell potential (EMF) is given by,
Where,
The cell potential value is positive in spontaneous cell and negative in nu in spontaneous cell.
Answer to Problem 19.32QP
The balanced overall cell reaction is,
Explanation of Solution
The Oxidation half cell reaction is,
The reduction half cell reaction is,
To sum the two half cell reactions and remove a electron to give a balanced overall cell reaction.
The balanced overall cell reaction is,
Want to see more full solutions like this?
Chapter 19 Solutions
General Chemistry - Standalone book (MindTap Course List)
- A voltaic cell is constructed using the reaction Mg(s) + 2H+(aq) Mg2+(aq) + H2(g) (a) Write equations for the oxidation and reduction half-reactions. (b) Which half-reaction occurs in the anode compartment, and which occurs in the cathode compartment? (c) Complete the following sentences: Electrons in the external circuit flow from the ________ electrode to the ______ electrode. Negative ions move in the salt bridge from the ______ half-cell to the ______ half-cell. The half-reaction at the anode is ____, and that at the cathode is _____.arrow_forwardIn principle, a battery could be made from aluminum metal and chlorine gas. (a) Write a balanced equation for the reaction thatwould occur in a battery using Al3+(aq) | Al(s) andCl2(g) | Cl(aq) half-cells. (b) Identify the half-reaction at the anode and at the cathode. Do electrons flow from the Al electrode when thecell does work? Explain. (c) Calculate the standard potential, Ecell, for the battery.arrow_forwardGive the notation for a voltaic cell whose overall cell reaction is Mg(s)+2Ag+(aq)Mg2+(aq)+2Ag(s) What are the half-cell reactions? Label them as anode or cathode reactions. What is the standard cell potential of this cell?arrow_forward
- A voltaic cell is constructed using the reaction of chromium metal and iron(II) ions. 2 Cr(s) + 3 Fe2+(aq) 2 Cr3+(aq) + 3 Fe(s) Complete the following sentences: Electrons in the external circuit flow from the ________ electrode to the ______ electrode. Negative ions move in the salt bridge from the ________ half-cell to the ______ half-cell. The half-reaction at the anode is _______ and that at the cathode is ________.arrow_forwardConsider a galvanic cell based on the following half-reactions: a. What is the expected cell potential with all components in their standard states? b. What is the oxidizing agent in the overall cell reaction? c. What substances make up the anode compartment? d. In the standard cell, in which direction do the electrons flow? e. How many electrons are transferred per unit of cell reaction? f. If this cell is set up at 25C with [Fe2+] = 2.00 104 M and [La3+] = 3.00 103 M, what is the expected cell potential?arrow_forwardFrom the information provided, use cell notation to describe the following systems: (a) In one half-cell, a solution of Pt(NO3)2 forms Pt metal, while in the other half-Cell, Cu metal goes into a.Cu(NO3)2 solution with all solute concentrations 1 M. (b) The cathode consists of a gold electrode in a 0.55 M Au(NO3)3 solution and the anode is a magnesium electrode in 0.75 M Mg(NO3)2 solution. (c) One half-cell consists of a silver electrode in a 1 M AgNO3 solution, and in the other half-cell, a copper Electrode in 1 M Cu(NO3)2 is oxidized.arrow_forward
- A voltaic cell is constructed in which one half-cell consists of a silver wire in an aqueous solution of AgNO3.The other half cell consists of an inert platinum wire in an aqueous solution containing Fe2+(aq) and Fe3+(aq). (a) Calculate the cell potential, assuming standard conditions. (b) Write the net ionic equation for the reaction occurring in the cell. (c) Which electrode is the anode and which is the cathode? (d) If [Ag+] is 0.10 M, and [Fe2+] and [Fe3+] are both 1.0 M, what is the cell potential? Is the net cell reaction still that used in part (a)? If not, what is the net reaction under the new conditions?arrow_forwardYou want to set up a series of voltaic cells with specific cell potentials. A Zn2+(aq, 1.0 M)| Zn(s) half-cell is in one compartment. Identify several half-cells that you could use so that the cell potential will be close to (a) 1.1 V and (b) 0.50 V. Consider cells in which the zinc cell can be either the cathode or the anode.arrow_forwardA voltaic cell is constructed from the following half-cells: a chromium electrode in chromium(III) sulfate solution and a lead electrode in lead(II) sulfate solution. The half-reactions are Cr(s)Cr3(aq)+3ePb2+(aq)+2ePb(s) Sketch the cell, labeling the anode and cathode (and the electrode reactions), and show the direction of electron flow and the movement of cations.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





