Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 83SCQ
Related questions
Question
2. Calculate the energy associated with the light emitted by Rubidium and Cesium
Transcribed Image Text:PP
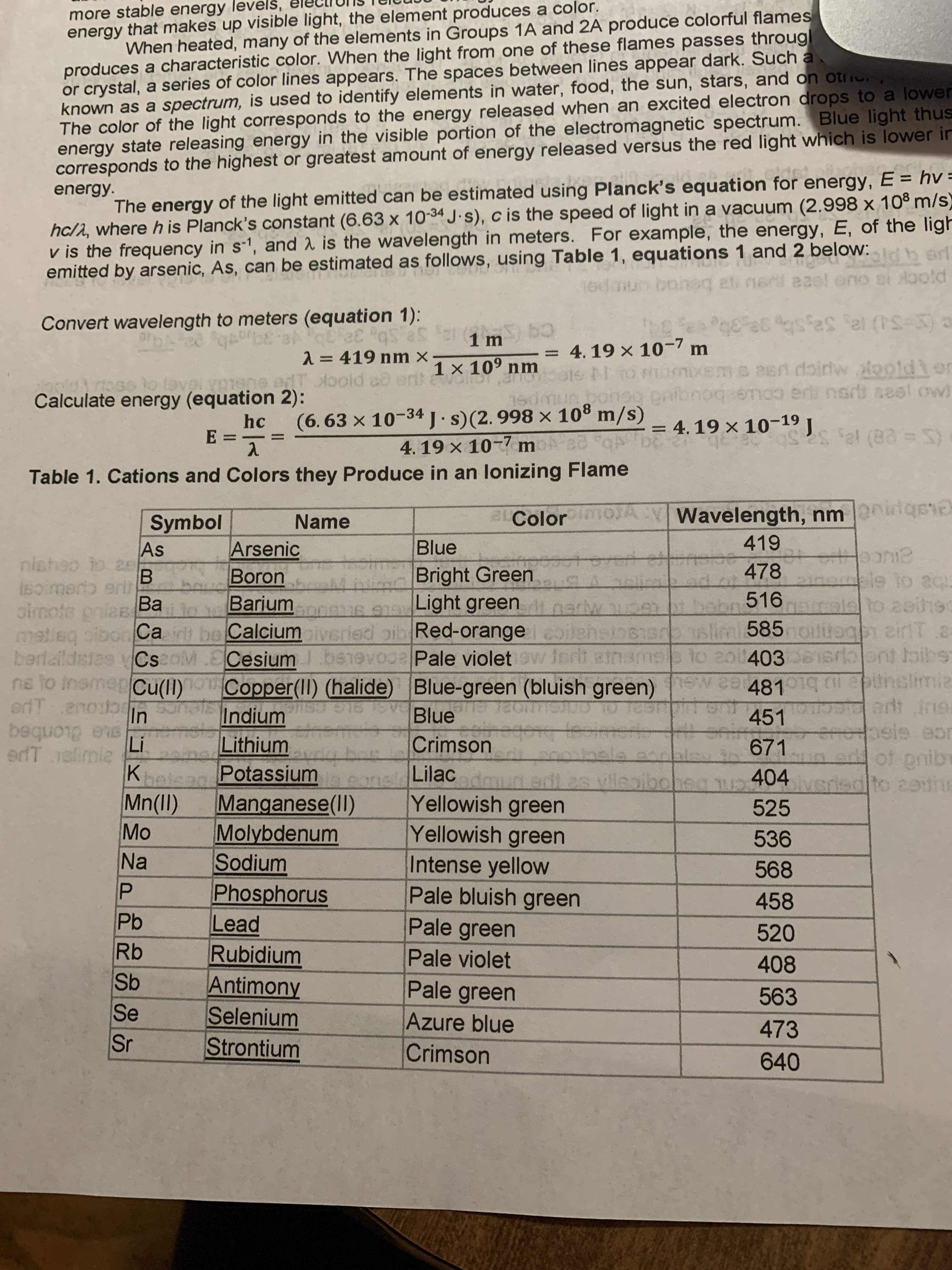
more stable energy levelš,
energy that makes up visible light, the element produces a color.
When heated, many of the elements in Groups 1A and 2A produce colorful flames
produces a characteristic color. When the light from one of these flames passes throug!
or crystal, a series of color lines appears. The spaces between lines appear dark. Such a.
known as a spectrum, is used to identify elements in water, food, the sun, stars, and on otic
The color of the light corresponds to the energy released when an excited electron drops to a lower
energy state releasing energy in the visible portion of the electromagnetic spectrum. Blue light thus
corresponds to the highest or greatest amount of energy released versus the red light which is lower in
The energy of the light emitted can be estimated using Planck's equation for energy, E = hv =
hc/1, where h is Planck's constant (6.63 x 10-34 J.s), c is the speed of light in a vacuum (2.998 x 108 m/s)
v is the frequency in s-1, and 2 is the wavelength in meters. For example, the energy, E, of the ligh
emitted by arsenic, As, can be estimated as follows, using Table 1, equations 1 and 2 below:
energy.
16d mun boheq et neri aael ono ai oold
34,
= 4. 19 × 10-7 m
Convert wavelength to meters (equation 1):
2= 419 nm x
1 × 10° nm
o e piock
(6.63 x 10-34 J·s)(2.998 × 108 m/s)
4. 19 × 10-7 m aa q
pnfbnoqeemoo
erli nert aeel ow
Calculate energy (equation 2):
hc
= 4. 19 × 10-19 J
%3D
E.
Table 1. Cations and Colors they Produce in an lonizing Flame
BLColor
Color moA V Wavelength, nm idqs
Name
Symbol
As
elabp
419
Arsenic
Boron
Bariumng
Blue
nisheo io ae
toime
478
B.
b erit
Bright Green
Light green
ainemple to 2G
516 pl
lim 585noilitegh zirlT.s
to 20i403 Isront oibs
to aoihsc
simots pnlase
matieg oibon Ca be Calciumiveried oib Red-orange coilensios1er
berlaildsies vCseoM.ECesium
ne to InsmepCu(Il)OCopper(II) (halide) Blue-green (bluish green) Sw ea 481 1g Cl eptinslmiz
अी aoe
Ba
benevo Pale violetw lerlt ainamso to 20i 403 1Sriaont taibs
Blue-green (bluish green) w e 481 1g Cl
Indium
BASI SIG Cse
Blue
451
gle BLonbeq
Lithium
Potassiumn
pele eor
of pnibe
404 sriedto 29the
Crimson
671
edT elimie
K.
Mn(1I)
ने
Manganese(Il)
Molybdenum
Lilac
26 viisoibol
Yellowish green
525
Yellowish green
Intense yellow
Pale bluish green
536
Na
Sodium
568
Phosphorus
Lead
458
Pale green
520
Rb
Rubidium
Pale violet
408
Sb
Se
Antimony
Selenium
Strontium
Pale green
Azure blue
563
473
Crimson
640

Transcribed Image Text:1. List the expected color of light and the corresponding wavelength (nm) associated with each cation
(use Table 1).
COLL C:
Rubidium, Rb* 0ale violet
G.CCUK
Ceen
anotelualsn suoy wone ihono ilut soS
edi st
Cesium, Cs
2. Calculate the energy associated with the light emitted by each of the elements above (sue equations
1 and 2).
Rubidium, Rb*
Cesium, Cs*
4 awominl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning