2. Fill in the blanks. symbol element capital atom lowercase two solid a) All mater is made up of tiny particles called an b) Most elements are found in the c) The short way of writing an element is called its state. d) A chemical symbol never has more than e) The first letter of a chemical symbol is always f) If a chemical symbol has more than one letter, the second letters are always letters. letter letters. g) Matter that is made up of only one kind of atom is called an
2. Fill in the blanks. symbol element capital atom lowercase two solid a) All mater is made up of tiny particles called an b) Most elements are found in the c) The short way of writing an element is called its state. d) A chemical symbol never has more than e) The first letter of a chemical symbol is always f) If a chemical symbol has more than one letter, the second letters are always letters. letter letters. g) Matter that is made up of only one kind of atom is called an
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 88AP
Related questions
Question
Help me with
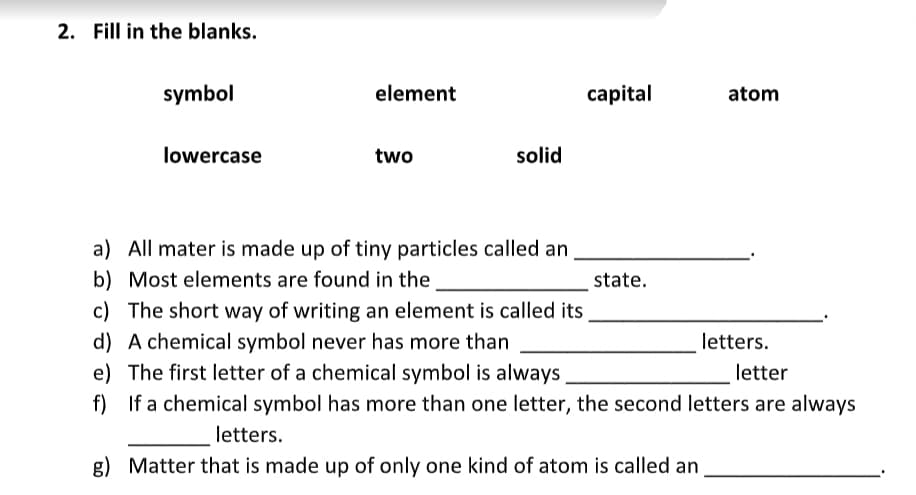
Transcribed Image Text:2. Fill in the blanks.
symbol
element
capital
atom
lowercase
two
solid
a) All mater is made up of tiny particles called an
b) Most elements are found in the
state.
c) The short way of writing an element is called its
d) A chemical symbol never has more than
e) The first letter of a chemical symbol is always
f) If a chemical symbol has more than one letter, the second letters are always
letters.
letter
letters.
g) Matter that is made up of only one kind of atom is called an
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning