2. From item no. 1, List 5 elements that exhibit paramagnetism and 5 elements that exhibit diamagnetism in its ground state. 3. Write the four quantum numbers of each electron in a nitrogen atom.
2. From item no. 1, List 5 elements that exhibit paramagnetism and 5 elements that exhibit diamagnetism in its ground state. 3. Write the four quantum numbers of each electron in a nitrogen atom.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 95AP
Related questions
Question
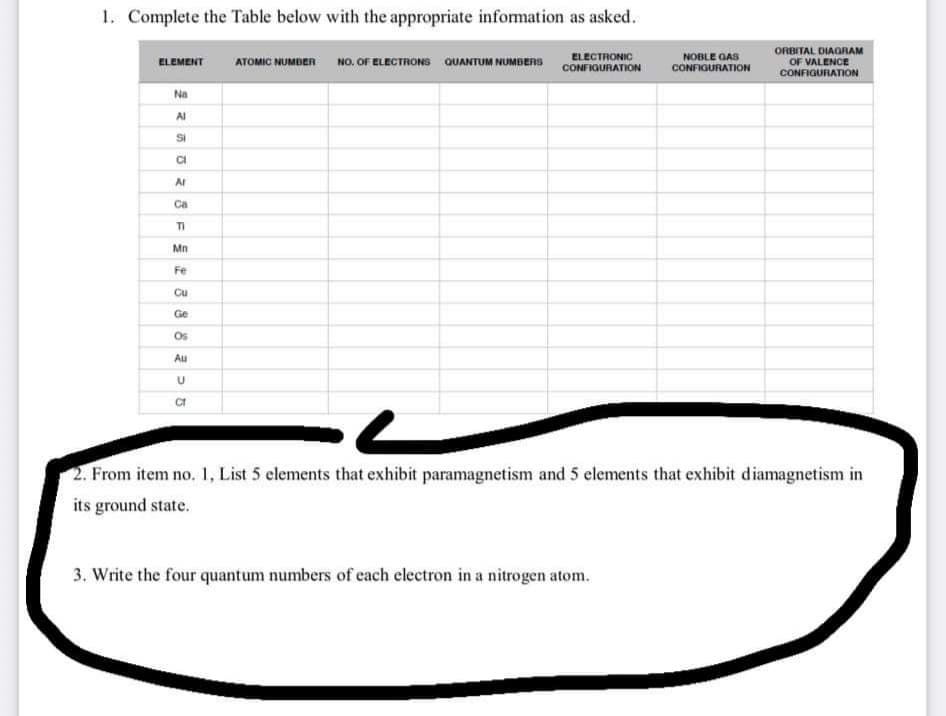
Transcribed Image Text:1. Complete the Table below with the appropriate information as asked.
NO. OF ELECTRONS QUANTUM NUMBERS
ELECTRONIC
CONFIQURATION
NOBLE GAS
CONFIGURATION
ORBITAL DIAGRAM
OF VALENCE
CONFIGURATION
ELEMENT
ATOMIC NUMBER
Na
Al
Si
AI
Ca
TI
Mn
Fe
Cu
Ge
Os
Au
2. From item no. 1, List 5 elements that exhibit paramagnetism and 5 elements that exhibit diamagnetism in
its ground state.
3. Write the four quantum numbers of cach electron in a nitrogen atom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning