Q: Fill in all H's and lone pairs in each compound. b. c--c а. С—С—с—С С. С —С—С d. C-Ĉ-N-C е.
A:
Q: d. H E Assign formal charges to each carbon atom in the given species. All lone pairs have been…
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3 sub-parts. Please resubmit the…
Q: Calculate the formal charge on the circled atoms in the following structures. formal charge:…
A: Formal charge calculation: We know that the formal charge can be expressed as follows --- Formal…
Q: 1) Identify any formal charges for the following structures (all lone pairs of electrons are shown):
A:
Q: Draw in all H's and lone pairs in each compound. a. C–C-CI b. C-C=0 С. С -С—N
A: There are 5 H's and 3 lone pairs on chlorine.
Q: Which of the following compounds has a labeled atom with a +1 formal charge? (All nonbonded electron…
A: Use the formula of formal charge . In written solution, F.C. is formal charge on the indicated…
Q: w the simplest set of curved arrows that shows how the structure on the left could be turned into…
A:
Q: Assign formal charges to each carbon atom in the given species. All lone pairs have been drawn in.…
A:
Q: Calculate the formal charge for each atom in the following HCOO- ion. H: C: O (single bond) : O…
A: As seen in the formula, the formal charge is proportional to the difference between the valence…
Q: Considering structures A-D, classify each pair of compounds as isomers, resonance structures, or…
A: Isomers are the compounds having molecular formula different structures. Isomers are various type,…
Q: Draw in all H atoms and lone pairs in each ion. -ÑCH3 b. =NH d. a. C. O
A: Total H-atoms are calculated when this skeletal structure converted into the condensed structure or…
Q: Identify the formal charge on each atom in the following H species. Assume that all valence…
A: The given molecule is represented as follows:
Q: Identify the following pair that may not be classified as resonance structures: CH, CH, A ... CH3…
A: Resonance is a delocalization of the pi electrons of a conjugated system.The structures obtained by…
Q: Compute the formal charge (FC) on each atom in the following structures.(a) Methane (CH4)
A: Valence electron of C = 4 Valence electron of H = 1 Formula to calculate Formal charge: Formal…
Q: For each of the following structures,1. Draw a Lewis structure; fill in any nonbonding electrons.2.…
A: Hello. Since your question has multiple sub-parts, we will solve the first three sub-parts for you.…
Q: Draw the contributing structure indicated by the curved arrow(s). Assign formal charges as…
A: resonance is the delocalization of electrons within certain molecules, ions. A molecule or ion with…
Q: formal charges: all of the atoms are 0 formal charges: sulfur and two oxygen atoms are 0, the other…
A: In case of SO4^2- many resonating structures can be drawn. But, we can also drawn only two…
Q: HC1 + (CH₂CH₂)₂0 CI + نے
A:
Q: Q2. Draw a zig-zag representation for each molecule below showing all valence electrons on atoms…
A:
Q: IO sa anion, C5H5-. Label all non-zero formal charges. (Hint: as the name suggests, the five carbons…
A: Resonance structure stabilized the molecule by dispersing the charge which makes it more stable then…
Q: In their neutral forms shown below, how many lone pairs are present in (a) (b) and (c) (a) (b) (c)
A: Given Neutral forms of compound.
Q: Draw the best resonance structure for cyanic acid HOCN . Be sure to include all lone pair…
A: We have to draw the best resonance structure for cyanic acid HOCN as follows in step 2 :
Q: Draw the contributing structure indicated by the curved arrows. Show all valence electrons and all…
A:
Q: Following the rule that each atom of carbon, oxygen, and nitrogen reacts to achieve a complete outer…
A:
Q: Indicate the strongest and the weakest C-H bond (identified by an arrow) in the molecule below. H H…
A: C- H bond that cannot be easily dissociable. Bond can be dissociated by providing high energy.
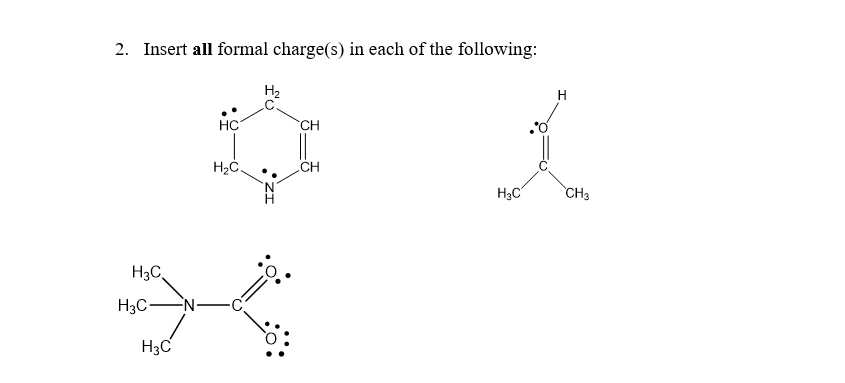
Q: 3. Fill in Necessary Lone Pair Electrons and Indicate formal charges in the following molecule where…
A: As per our company guidelines, we can answer one question. Kindly repost the other questions
Q: Draw the contributing structure indicated by the curved arrow(s). Assign formal charges as…
A: A resonance structure is formed when the lone pair of electrons or pi-bond electrons of a molecule…
Q: 2. Determine the formal charges on S and C in the structure below, then draw a better structure in…
A:
Q: Calculate the formal charge on each second-row atom.
A: Formal charge is the charge present on the bonded atom in the covalent molecule. Formal charge can…
Q: Draw the contributing structure indicated by the curved arrow(s). Assign formal charges as…
A: The structure given is,
Q: Compute the formal charge (FC) on each atom in the following structures. H3N¬BH3
A: The formal charge present on atom could be calculated using the formula, Formal charge=Valence…
Q: Convert each condensed formula to a Lewis structure. a.CH3(CH2)4CH(CH3)2 b. (CH3)3CCH(OH)CH2CH3 c.…
A: a,b) Please find below the Lewis structure of a and b
Q: Assign a formal charge to each atom of the above. OCI = 2', 0- 1 O C 0,0= 0 O CI - 2", O = 1 O CI-…
A:
Q: Determine the geometry around all second-row elements in each compound drawn as a Lewis structure…
A:
Q: Following the rule that each atom of carbon, oxygen, and nitrogen reacts to achieve a complete outer…
A: The octet rule is a chemical thumb rule that says that the main group elements tend to bond with…
Q: Solve for the formal charge of the central atom for each of the following: a.N(CH3)4 b.-CH3 c. .CH3…
A: Charge on central metal atom: Charge on metal occur when element looses or gain electron within…
Q: Which molecule contains carbon with a negative formal charge? CO CO2 H2CO CH4
A:
Q: Evaluate the molecule below and assign formal charges to each atom. :0: C 17 1) Formal charge of…
A: Step by step solution are below
Q: Specify the formal charge at each of the labeled atoms, a - c, in each of the following structures.…
A: Formal charge of an atom in a molecule is the charge assigned to it, assuming that all chemical…
Q: Calculate the formal charge on each indicated atom (a and b) in the given molecules. All lone pairs…
A: The formal charge on each indicates atom has to be given,
Q: CH,CH;CH, H3C CH3 H,CH;C CH3 H,CH,C CH;CH;CH3
A:
Q: 5. Calculate the formal charges on the indicated atoms in each compound below. :0:- B. C. D. :C1-…
A: Formal charge :- It may be defined as the difference between thenumber of valence electrons in an…
Q: 10. Write the structures obtained when electrons move as indicated by the curved arrows in the…
A:
Q: Convert each condensed formula to a Lewis structure. a. CH3(CH2)4CH(CH3)2 b. (CH3)3CCH(OH)CH2CH3 c.…
A: Since we answer upto 3 sub-parts, we'll answer first three. Please resubmit the question specifying…
Q: Assign formal charges to each carbon atom in the given species. All lone pairs have been drawn in. H…
A:
Q: Calculate the formal charge for each atom that is not carbon or hydrogen in the following molecules.
A:
Q: Q3. Circle the following pairs of structures that do not constitute resonance structures. H3C.CH3…
A: Interpretation- To circle all the pairs which do not have resonance in their structures -…
Q: Draw the contributing structure indicated by the curved arrow(s). Show all valence electrons and all…
A: The contributing structure of the given molecules is as follows:
Q: (1) НзС—N3N: (2) H2C-C=C-CH2 нн :0: :0: (4) Н-С OCH2 (3) Н-С 0-H
A: “Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Compute the formal charge (FC) on each atom in the following structures.(a) Methane (CH4)Label each non-hydrogen atom in the structure below with its formal chargecan someone explain how Molecule B has a tertiary carbon ? Im having troubl with secondary and tertiary i tried to calculate the formal charges thinking that may have something to do with it but I ended up getting the wrong formal charge for molecule B, so now I'm confused.
- Write the best Lewis or Kekulé structure (showing all lone pairs of electrons, formal charges, and appropriate geometry – use wedge-dash where needed) for each of the following species given as condensed formulas: a) NH2- b) PO3H3 c) H2COH+ d) NaBH3CN e) C6H5MgBr f) CH3AlCl2Draw the missing lone-pair electrons and assign the missing formal charge on N or O for the following H-C-O-HCompute the formal charge (FC) on each atom in the following structures. H3N¬BH3
- Follow the curved arrows to draw a second resonance structure for eachspecies.Draw in all hydrogens and lone pairs on the charged carbons in each ion ?Considering structures A–D, classify each pair of compounds as isomers, resonance structures, or neither: (a) A and B; (b) Aand C; (c) A and D; (d) B and D



