2. Joey was tasked to ensure that an enzyme responsible for keeping his species from extinction remains biologically active. To do so, he needs to store it in a buffer solution with a pH of 10 00. Unfortunately, only three buffer systems are available in their laboratory Buffer System Weak Acid Component K Conjugate Base Salt KYA MUH TWO A. In what buffer system (KYA, MUH, or TWO) will Joey store the enzyme? In your solution sheet, show calculations and briefly explain to support your answer B. Write the equilibrium reaction involved in the buffer system. Write your answer on your solution sheet C Calculate the amount (in mL) of the 1.75 M stock solution (pH 7.00) of the same buffer system needed to prepare 250 mL of a 0.550 M buffer pH 7.00. Benzoic acid, C,H,COOH 6.25 x 10 NaC,H,COO Boric acid, H,BO, 5.81 x 107 NaH BO Hydrogen cyanide, HCN 620 X 10 10 NaCN
2. Joey was tasked to ensure that an enzyme responsible for keeping his species from extinction remains biologically active. To do so, he needs to store it in a buffer solution with a pH of 10 00. Unfortunately, only three buffer systems are available in their laboratory Buffer System Weak Acid Component K Conjugate Base Salt KYA MUH TWO A. In what buffer system (KYA, MUH, or TWO) will Joey store the enzyme? In your solution sheet, show calculations and briefly explain to support your answer B. Write the equilibrium reaction involved in the buffer system. Write your answer on your solution sheet C Calculate the amount (in mL) of the 1.75 M stock solution (pH 7.00) of the same buffer system needed to prepare 250 mL of a 0.550 M buffer pH 7.00. Benzoic acid, C,H,COOH 6.25 x 10 NaC,H,COO Boric acid, H,BO, 5.81 x 107 NaH BO Hydrogen cyanide, HCN 620 X 10 10 NaCN
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 4RQ: A good buffer generally contains relatively equal concentrations of weak acid and conjugate base. If...
Related questions
Question
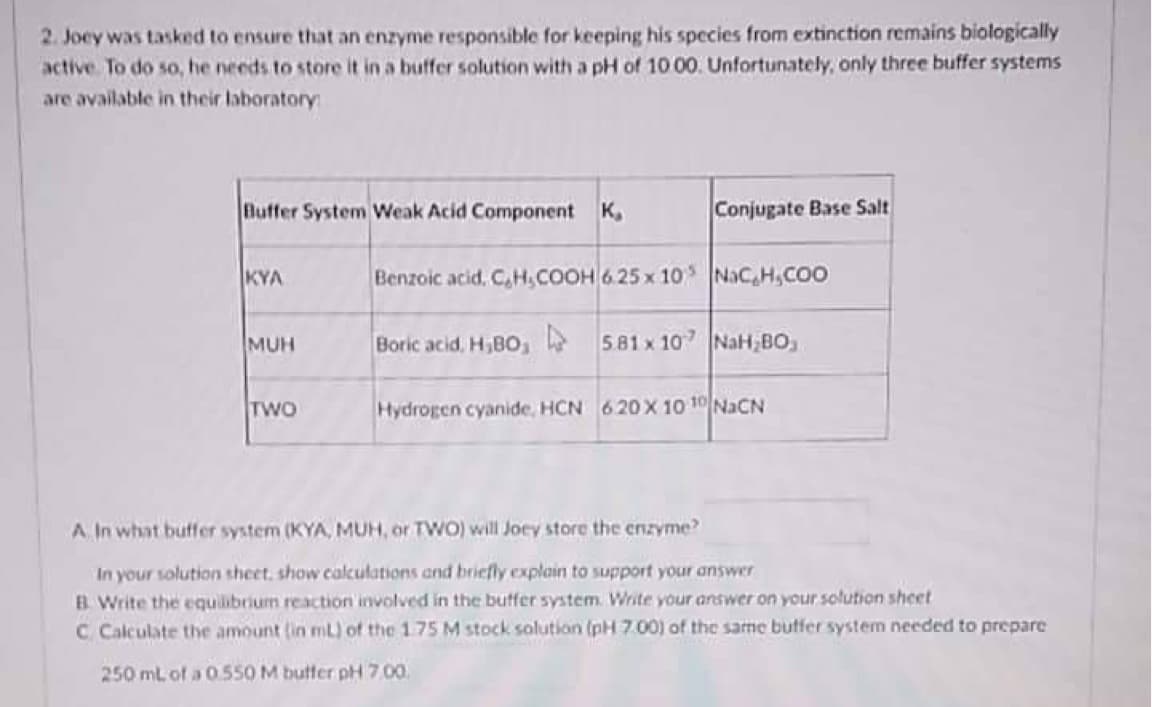
Transcribed Image Text:2. Joey was tasked to ensure that an enzyme responsible for keeping his species from extinction remains biologically
active. To do so, he needs to store it in a buffer solution with a pH of 10 00. Unfortunately, only three buffer systems
are available in their laboratory
Buffer System Weak Acid Component K
Conjugate Base Salt
KYA
Benzoic acid, C,H,COOH 6.25 x 10 NaC,H,COO
Boric acid, H;BO, 5.81 x 107 NaH BO
Hydrogen cyanide, HCN 620 X 10 10 NaCN
MUH
TWO
A. In what buffer system (KYA, MUH, or TWO) will Joey store the enzyme?
In your solution sheet, show calculations and briefly explain to support your answer
B. Write the equilibrium reaction involved in the buffer system. Write your answer on your solution sheet
C Calculate the amount (in mL) of the 1.75 M stock solution (pH 7.00) of the same buffer system needed to prepare
250 mL of a 0.550 M buffer pH 7,00.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning