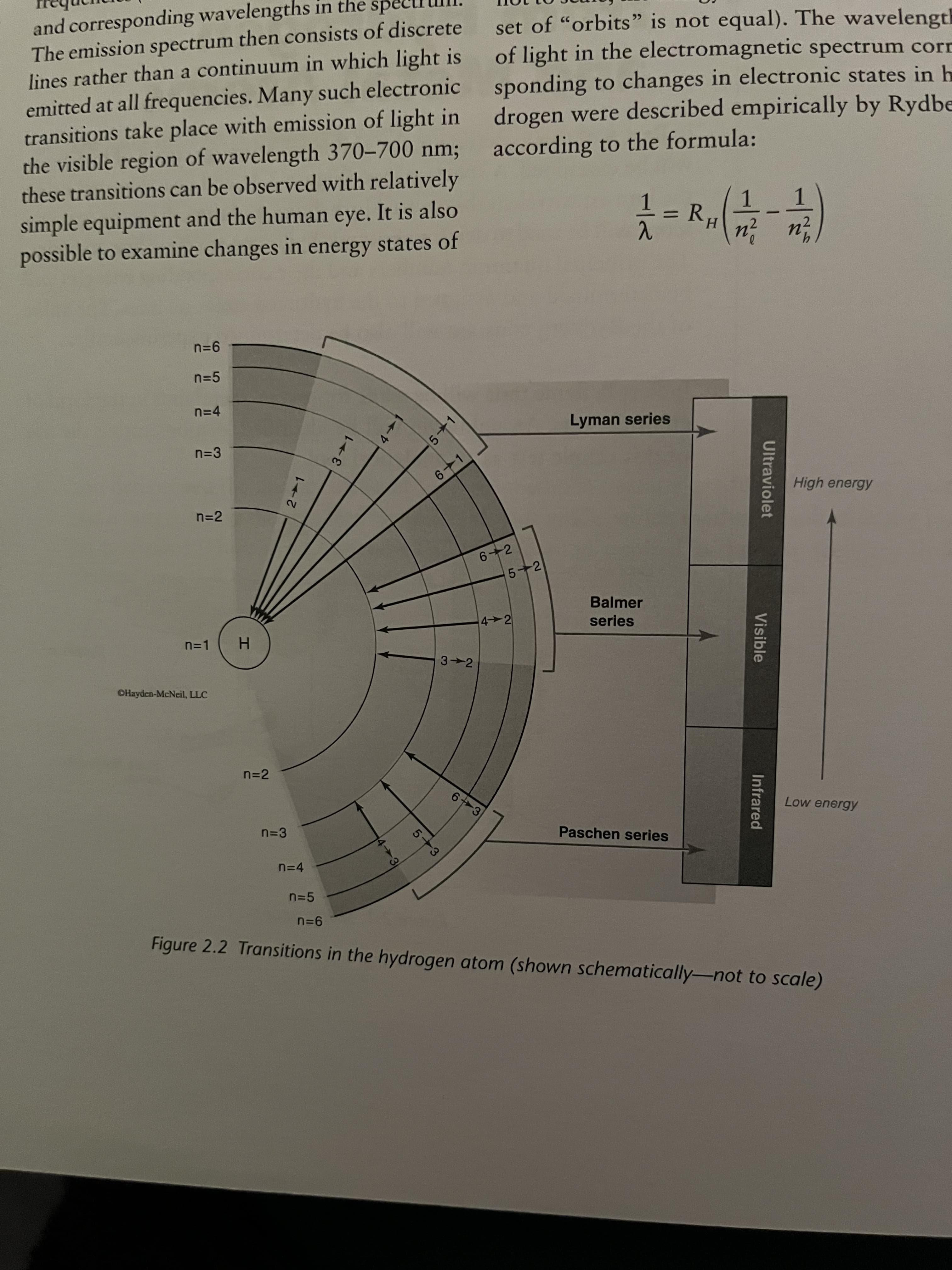
2. Rank the following three transitions in the hydrogen atom in terms of lowest to highest wave- length, energy, and frequency: 6 → 5, 3 → 1, 7-→ 3. Note that the energy levels in Figure 2.2 are shown schematically, so you must calculate the energy difference between any two levels in order to answer this question. Show all calculations below or on the following page. Wavelength: Energy: Frequency:
2. Rank the following three transitions in the hydrogen atom in terms of lowest to highest wave- length, energy, and frequency: 6 → 5, 3 → 1, 7-→ 3. Note that the energy levels in Figure 2.2 are shown schematically, so you must calculate the energy difference between any two levels in order to answer this question. Show all calculations below or on the following page. Wavelength: Energy: Frequency:
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.47QP: A particular transition of the rubidium atom emits light whose frequency is 3.84 1014 Hz. (Hz is...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
Transcribed Image Text:Ultraviolet
Visible
Infrared
5 1
4-1
3 1
2 1
4.
3
and corresponding wavelengths in the
The emission spectrum then consists of discrete
lines rather than a continuum in which light is
emitted at all frequencies. Many such electronic
transitions take place with emission of light in
the visible region of wavelength 370-700 nm;
these transitions can be observed with relatively
sp
set of "orbits" is not equal). The wavelength
of light in the electromagnetic spectrum corr
sponding to changes in electronic states in h
drogen were described empirically by Rydbe
according to the formula:
simple equipment and the human eye. It is also
possible to examine changes in energy states of
1.
R.
1.
1.
H.
n=5
n=4
Lyman series
n=3
6-1
High energy
n=2
6 2
5+2
Balmer
4 2
series
n=1
H.
3 2
OHayden-McNeil, LLC
n-2
6 3
Low energy
n=3
Paschen series
n=4
Figure 2.2 Transitions in the hydrogen atom (shown schematically-not to scale)

Transcribed Image Text:2. Rank the following three transitions in the hydrogen atom in terms of lowest to highest wave-
length, energy, and frequency: 6→ 5, 3 → 1, 7 → 3. Note that the energy levels in Figure 2.2
are shown schematically, so you must calculate the energy difference between any two levels
in order to answer this question. Show all calculations below or on the following page.
Wavelength:
Energy:
Frequency:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning